H-3
Note: Some of the acids are slightly hygroscopic. Therefore you should handle your unknown acid
in such a way, as to minimize exposure to the atmosphere when weighing out the samples.
The volume of water added to dissolve the acid does NOT affect the volume of NaOH solution
required to reach the equivalence point, because it is the moles of acid present in the weighed
sample that determines the moles of titrant required.
Since you do not know the pH at the equivalence point for your titration, you will need to use a pH
meter for the first titration. Once this titration is complete, you will need to plot the graph of pH
versus volume of NaOH added in order to determine (graphically) the pH of the equivalence point.
This will allow you to select a suitable indicator that will be used to do several more regular
titrations of your unknown acid.
NOTE:
You will be required to plot your data from the pH titration on graph paper as
you perform the titration. Therefore, come prepared to plot the graph during the
laboratory session by labeling, in advance, the axes on a piece of 1.0 mm grid
graph paper in the following manner: pH scale on the y-axis (short axis) from 0-
14 and the volume of NaOH on the x-axis (long axis) from 0 – 48 mL.
ADVANCE STUDY ASSIGNMENT
1.
Review the previous information on acid-base chemistry.
2.
Review instructions on the use of pH meters. (pages 25 - 27)
3.
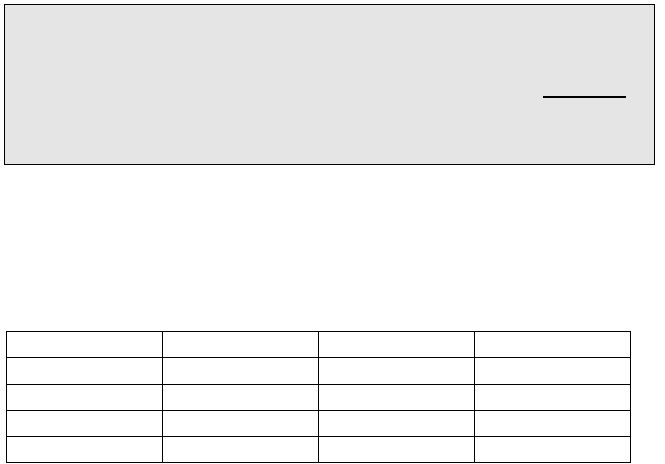
Fill in the table
pH
[H3O
+
]
[OH
-
]
pOH
13.134
1.66 x 10
-2
M
1.9 x 10
-8
M
11.8
4.
Explain why the unknown weak acid is completely neutralized by the added NaOH in a
titration. Use Le Chatelier’s Principle and the weak acid dissociation equilibrium to explain.