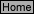H-2
This is a very fast reversible reaction in which the reverse reaction is very much favored under
normal laboratory conditions (as can be inferred by the equilibrium constant K
w
for this reaction).
The equilibrium constant K
w
is the product of the H3O
+
ion and OH
-
ion concentrations and is
equal to 1.00 x 10
-14
(a constant) at 25
C. Changing the concentration of one ion, results in a
stress in the equilibrium of the water autoionization reaction resulting in a almost instantaneous
change in the other ion. The product of the two ion concentrations must equal 1.00 x 10
-14
. This
is a very small number, hence, typically the concentration of H3O
+
or OH
-
are often much less
than 1 M and may vary considerably (0.1 to 1x 10
-13
M for instance).
It is often inconvenient to plot changes in the concentration of H3O
+
(aq)
directly, instead, a
logarithmic scale known as the pH scale is used to show changes in the concentration of H3O
+
(aq)
.
pH is defined as the negative logarithm of the [H
+
] or [H3O
+
] (you can think of the “p” in pH as
being equivalent to the function “-log”. We can similarly define pOH as -log[OH
-
] or pK
w
as K
w
)
= –log(1.00 x 10
-14
) = 14.000 (a constant).
Another useful expression may be derived by taking the negative logarithm of the K
w
expression
is shown below:
K
w
= [H3O
+
][OH
-
] = 1.00 x 10
-14
-log(K
w
) = -log([H3O
+
][OH
-
]) = -log(1.00 x 10
-14
)
pK
w
= -log([H3O
+
]) + -log([OH
-
]) = 14.000
pK
w
= pH + pOH = 14.000
EXPERIMENTAL METHOD
The acids used as unknowns in this experiment are solids and have molar masses in the range 50-
250 g mol
-1
. To determine the moles of acid present, you must carry out at least three titrations.
The first titration will utilize a pH meter. The other two, (three, if time permits), will employ an
indicator (properly chosen using the information obtained from the pH titration curve). You will
evaluate:
1.
the number of moles of base required to neutralize a weighed sample of the unknown
acid
2.
the molar mass of the acid
3.
the acid dissociation constant of your unknown acid
You will weigh out a known mass of a solid acid into a container, dissolve it in water, and then
titrate it with a previously standardized NaOH solution.