H-4
5.
A student weighed out 1.111 g of an unknown acid into a beaker and dissolved it using
100.0 mL of deionized water. The titration using NaOH and a pH meter found that 25.52
mL of NaOH was required to reach the equivalence point. How would the equivalence
volume of NaOH, change if the 1.111 g sample had been dissolved in 102.0 mL, or in 200.0
mL of deionized water?
Answer: No change because the moles of acid titrated is unchanged.
6.
If the NaOH used by you in Problem 5 (above) was 0.5555 M, what molar mass should be
reported for the unknown acid?
Answer: 78.37 g/mol
7.
How would you select an indicator that should be used for replicate titrations?
8.
Refer to figures 1 and 2 in experiment G to identify four differences between the titration
curve for a 0.50 M HA (weak acid) and the titration curve for 0.50 M HCl, each with 0.50 M
NaOH,
Answer: For the weak acid titration: (1)starting pH is larger (2) equivalence point pH > 7,
whereas for the strong acid pH = 7 at the equivalence point. (3) smaller jump in pH near
equivalence point (4) Initial pH change is significant.
REAGENTS AND APPARATUS
pH meter with electrode
buret (1
only)
standard buffer solutions: pH 4.00 and pH 7.00
indicator solutions (see below)
unknown acid samples
0.25 M HCl (hydrochloric acid)
0.2xxx M NaOH (standardized sodium hydroxide)
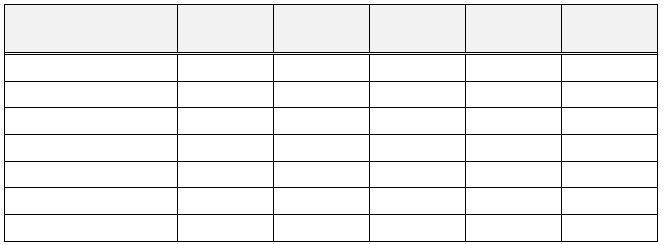
List of Indicators
Indicator
pH Range
pK
in
Acid
Base
Volume of
Indicator
bromocresol green
3.8 - 5.4
4.7
yellow
blue
5 drops
methyl red
4.2 - 6.2
5.0
red
yellow
5 drops
bromothymol blue
6.0 - 7.6
7.1
yellow
blue
8 drops
cresol red
7.2 - 8.8
8.2
yellow
magenta
4 drops
thymol blue
8.0 - 9.6
8.9
yellow
blue
10 drops
phenolphthalein
8.0 - 9.8
9.4
colorless
pink
3 drops
thymolphthalein
9.5 – 10.5
9.9
colorless
blue
10 drops