N-22
B.
Calculate ?H
10
from your value of ?H
solution
per mol of Na2SO
4
10 H2O
(s)
and the literature
value for ?H°
11
of -4.43 kJ/mol. Include the appropriate net ionic equations. (0.4 marks)
C.
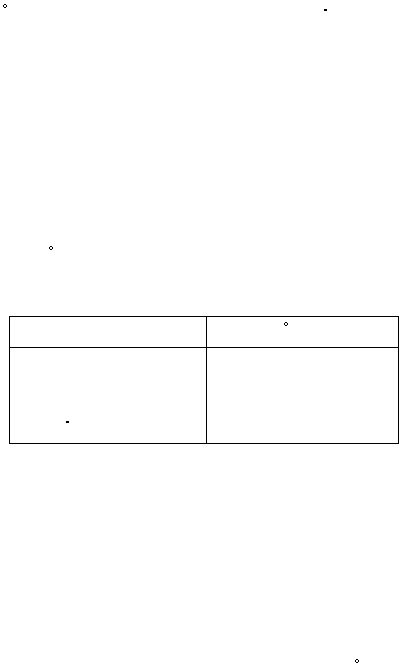
Use the following ?H
f
values to calculate the theoretical ?H°
rxn
of equation (10):
(0.5 marks)
compound
?H
f
, kJ/mol
Na2SO
4(s)
-1387.1
H2O
(l)
-285.8
Na2SO
4
10 H2O
(s)
-4327.3
D.
Calculate the percent error between your experimental value for ?H
rxn
and the theoretical
value? (0.2 marks)