N-21
D.
Using the literature value on your data sheet, determine the percent error in your
experimentally determined value of ?H
f
of H2O
(l)
. (0.2 marks)
Part II. Enthalpy of reaction, ?H
rxn
, between Na2SO
4
and water to form Na2SO
4
10 H2O
A.
Determination of ?H
solution
of Na2SO
4
10 H2O
(s)
(equation 12): (Your graph for this part must
be attached.)
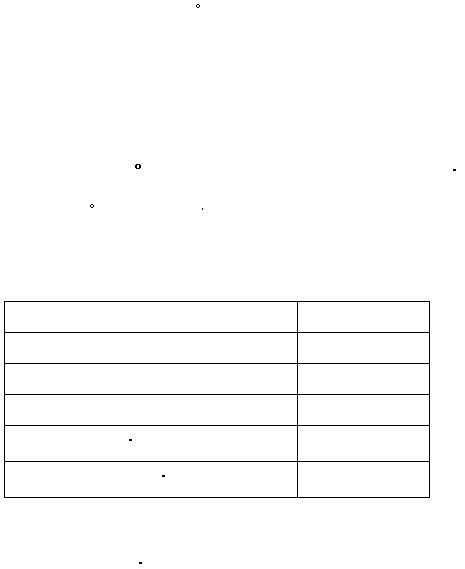
Results
Volume of water
mL
Mass of salt
g
?t
°C
Heat absorbed by water
J
Amount of Na2SO
4
10 H2O
(s)
moles
?H
12
per mol of Na2SO
4
10 H2O
kJ/mol
Show detailed calculations for the heat of surroundings, and ?H
12
, the
?H
solution
per mol of Na2SO
4
10 H2O. Include the calculations for ?t. (1.0 mark)