N-23
Part III. Heat of neutralization
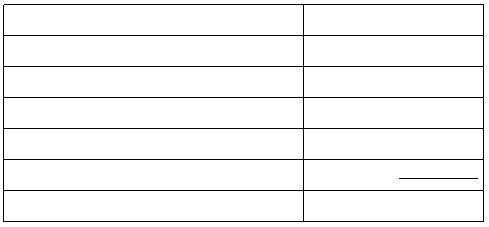
Results (part III a)
Volume of HCl
mL
Volume of NaOH
mL
Assumed volume of water
mL
?t
°C
Heat absorbed by water
J
Amount of limiting reagent (specify reagent)
mol
?H
13
per mol of water formed
kJ/mol
A.
Write the balanced molecular equation for the neutralization reaction of HCl and NaOH. (0.1
mark)
B.
Calculate the heat absorbed by the water in the calorimeter due to this reaction.
(0.2 marks)
C.
Calculate the ?H
neutralization
in kJ/mol of water formed. (0.5 marks)