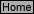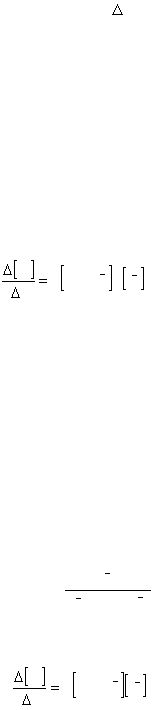
S-3
quantitatively determine. You will determine values of
t, corresponding to the time it takes to form a
fixed amount of I2, as a function of initial reactant concentrations.
As the concentration of the reactants increases, there will be less space between the reactant molecules.
When there is less space between reactant molecules, the number of collisions between reactant
molecules will increase, with a corresponding increase in the rate of reaction. This means that the rate of
reaction should be proportional to the concentration of reactants raised to some power, that is, rate = k
[S2O
8
2-
]
m
[I
-
]
n
, so that:
(3)
In this reaction, “m” and “n”, the exponents to which the concentration of reactants are raised, describe
the order of the reaction. The summation of m and n gives the overall order of the reaction. The
proportionality constant k is called the rate constant. You should note that its symbol is a small letter "k"
and it should not be confused with a capital K, which is the symbol for the equilibrium constant.
A common error is to write the rate law from the stoichiometry of the reaction, that is, to set n and m
equal to the coefficients in the balanced chemical equation as you would in writing the equilibrium
constant expression. For example, from equation (1) the equilibrium constant is:
K =
]
O
S2
[
]²
I
[
]
I
[
]
SO
[
2
8
2
2
2
4
In the same way, it is tempting to set the rate equation:
This would be correct only if the reaction was a single step reaction that proceeds by the simultaneous
collision between two I
-
ions, and one of S2O
8
2-
ion. Experiments have shown such termolecular
collisions are extremely rare, and that in general, m and n do not necessarily equal 1 and 2 respectively.
In general, there is no relationship between the overall stoichiometric equation and the rate
2
2
8
2
2
I
O
S
k
t
I
n
m
2
8
2
2
I
O
S
k
t
I