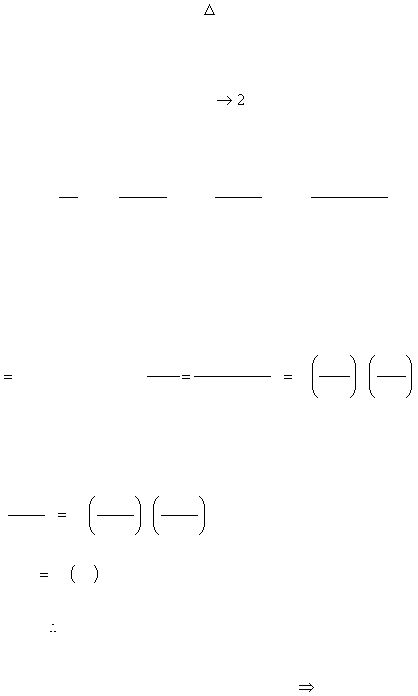
S-4
law. This means the rate law cannot be predicted from the overall stoichiometric equation and must be
established experimentally.
II.
Experimental Method for the Determination of the Rate Law
A general method for finding the order of a reaction is to mathematically solve for the values of
the exponents using the ratio method:
A series of concentration values and
t values can be substituted in the above expression and
then simultaneous equations can be solved to determine n and m. Consider a hypothetical
reaction:
A + 2B
C + D
(4)
Suppose we did a series of measurements, and found that at 296 K:
run
[A] (M)
[B] (M)
Rate (M s
-1
)
#1
0.010
0.010
0.010
#2
0.010
0.019
0.036
#3
0.017
0.015
0.038
Applying this equation to runs 1 and 2 will allow us to solve for the exponent, n:
And 1.9² = 3.61
n = 2
Or using logs at this final stage: log(3.6) = nlog(1.9)
0.556 = n(0.279)
n
1
2
m
1
2
n
1
m
1
n
2
m
2
1
2
n
m
]
B
[
]
B
[
]
A
[
]
A
[
]
B
[
]
A
[
]
B
[
]
A
[
rate
rate
and
]
B
[
]
A
[
Rate
:
If
n
m
010
.
0
019
.
0
010
.
0
010
.
0
010
.
0
036
.
0
n
9
.
1
6
.
3