S-2
I. The Rate Law
The speed or rate of a chemical reaction is defined as the change in concentration of reactant(s) or of
product(s) per unit of time,
t. Consider a solution containing 1.000 M of S2O
8
2-
which begins to react
with iodide at some initial time t = 0. Suppose that after 10 sec, the concentration of S2O
8
2-
has
decreased to 0.980 M. The rate of disappearance of S2O
8
2-
is given by the ratio of the change of
concentration of S2O
8
2-
to the change in time. Thus:
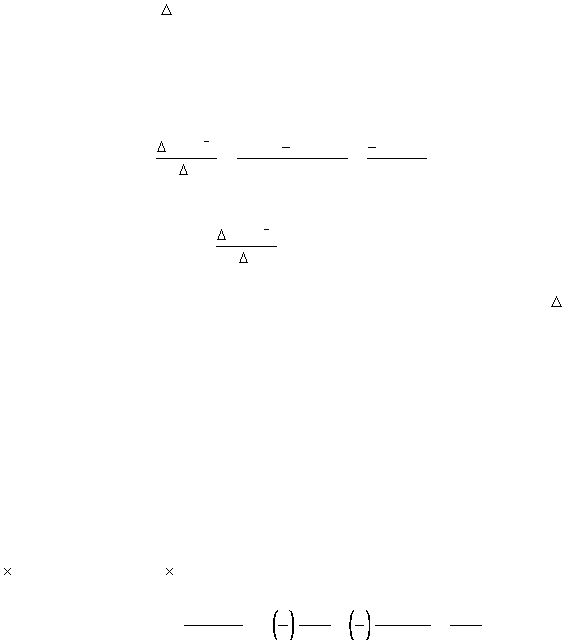
[S2
]
O²
t
8
=
s
10
M
)
000
.
1
980
.
0
(
=
0.
020
10
M
s
= -2.0x 10
-3
M s
-1
so the rate is:
rate = -
[S2
]
O²
t
8
= 2.0 x 10
-3
M s
-1
Note that the minus sign is necessary since the difference between the final [S2O
8
2-
] after
t and the
initial [S2O
8
2-
] is -0.0020 M and it is convenient and conventional to express the rate as a positive
number. Determining rates shortly after the reaction begins is known as rate determination by the
method of initial rates. This concept is discussed in much more detail with examples in your
textbook. The idea behind this method is to determine the instantaneous rate of reaction before the
concentrations of the initial reactants have changed significantly or before there is a buildup of products.
In this example, the change in reactant concentration is very small, less than 1%.
The stoichiometry of equation (1) tells us that if 0.0020 M S2O
8
2-
react, then
2
0.0020 M I
-
react and 2
0.0020 M SO
4
2-
and 0.0020 M I2 are formed. Thus:
rate
=
D[
S2
O²
8
]
Dt
=
1
2
D[
I
]
Dt
=
1
2
D[
SO
4
2
]
Dt
=
D[I
2
]
Dt
(2)
The rate of this chemical reaction can be expressed either in terms of the rate of disappearance of iodide
anion or peroxydisulfate ion (S2O
8
2-
), or by the rate of formation of iodine or the sulfate anion. It is only
necessary to determine the change in concentration of one of the reactants or products to know the
change in concentration of the other species. In this experiment, I2 is the compound that is easiest