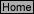![??M half-cell; Ecell = 0.059 V ?-cell? What is the corresponding [Cu2+]? Answer: - 0.282 V; 9.40 x 10-3 M esRED WIRECathodeAnode](./2002ExpR_Files/background9.jpg)
R-9
The position of this equilibrium lies far to the right. The equilibrium constant of reaction (15), referred to
as the formation constant (K
f
) or the stability constant (Kst) of the complex ion, is:
K
f
=
]
][NH
[Cu
]
]
)
[Cu(NH
4
3
2
2
4
3
(16)
Using Cu
2+
(1.00 M)/Cu as the reference half-cell, you will measure the cell potential, and obtain the
copper ion concentration at equilibrium by applying the Nernst equation. You will then calculate the
equilibrium concentrations of the other species in equation (15) from the stoichiometry of the reaction and
your knowledge of the initial amounts of reagents mixed together. From this information, you will be able
to calculate the formation constant of the complex ion using the K
f
relation above.
The following diagrams show the set up for this experiment.
ADVANCE STUDY ASSIGNMENT
1.
A voltaic cell was set up as follows: Ag/Ag
+
(0.10 M)//Ag
+
(1.00 M)/Ag. Use the Nernst
equation to determine the potential of each half-cell. Which half-cell is the anode? Which is the
cathode? Now use the equation E
cell
= E
red
+ E
ox
to determine the potential of the cell. Answer: anode is 0.10
2.
For the following reaction:
2 Ag
+
(aq)
+ Cu
(s)
2 Ag
(s)
+ Cu
2+
(aq)
a) what is the potential of the cell at equilibrium?
Answer: 0.000 V
b) what is the standard potential of the cell at equilibrium?
Answer: 0.458 V
3.
The measured potential of the following cell was 0.0600 V:
Cu/Cu
2+
(unknown concentration)//Cu
2+
(1.00 M)/Cu.
The Cu
2+
(1.00 M)/Cu reference half-cell was determined to be the cathode. Assuming the standard half-cell pot
0.059 V
AgNO3
(
1.0 M
)
AgNO3
(0.10 M
)
0.0010M
0.
00010M
Ag
+
Ag
+
0.010 M
Ag
+
Lin
es r
ep
r
esent
f
ilter
p
ap
er
salt b
rid
g
F
i
l
ter Paper
Voltmeter
BLACK WIRE
K
NO3
(1.
0
M
)
Si
lver Metal
(
stri
p)
Sil
ver Metal
(strip)
TOP V
IE
W
1.0 M
Ag
+
SIDE V
IEW
0.10 M
Ag
+
1.0
M
Cu²
+
1.0 M
CuSO
4
NH3
R
XN
RXN
C
uSO
4
NaOH
KNO3