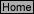![?3+] was determined to be less than 10-7 M. What is the concentration of [MCl2]+(aq) at equilibrium? Answer: 5.0 x 10-3 M ???](./2002ExpR_Files/background10.jpg)
R-10
4.
When 1.0 mL of 0.10 M CuSO
4
was added to 4.0 mL of 2.0 M NaOH, the product formed in
the reaction was Cu(OH)
2(s)
. From the measured potential of the cell, the calculated
concentration of the copper ion in solution was determined to be less than 10
-7
M. Calculate the
concentration of the OH
-
remaining in the solution after reaction?
Answer: 1.56 M
5.
In order to study the complex ion equilibrium: M
3+
(aq)
+ 2 Cl
-
(aq)
[MCl2]
+
(aq)
5.0 mL of 0.010 M M
3+
was added to 5.0 mL of 1.0 M NaCl. From the measured cell potential, the calculated
REAGENTS AND EQUIPMENT
voltmeter with leads
1.00 M KNO3 (potassium nitrate)
filter paper
24-well polystyrene plate
Ag, Cu wires or strips
0.100 M CuSO
4
(copper(II) sulfate)
1.00 M NaOH (sodium hydroxide)
1.00 M CuSO
4
(copper(II) sulfate)
AgNO3(silver nitrate)(1.00 M, 1.00 x 10
-1
M, 1.00 x 10
-2
M, 1.00 x 10
-3
M, 1.00 x 10
-4
M)
EXPERIMENTAL PROCEDURE
In the instructions that follow, the half-cells to be made up will be indicated schematically, for example:
Ag/Ag
+
(0.10 M)
This describes a half-cell consisting of a silver electrode placed in a 0.10 M silver nitrate solution. When
this notation is used to describe a complete cell, the cell notation is written in the direction of
spontaneous electron flow through the external circuit. For example, in the electrochemical cell
constructed from two half-cells: Zn/Zn
2+
(1.00 M) and Cu/Cu
2+
(1.00 M), the reduction potential of the
copper half-cell is more positive and this half-cell will be the cathode. The zinc half-cell will be the
anode where electrons are produced (Zn
(s)
Zn²
+
(aq)
+ 2 e
-
) so the electron flow in the external circuit
is from zinc to copper. The cell will be written as:
Zn/Zn
2+
(1.00 M)//Cu
2+
(1.00 M)/Cu (where the symbol // represents a salt bridge)