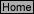R-8
We will use experimental values of the redox potential for this reaction to determine K
eq
and compare it
with the theoretical value. The reference half-cell will be Cu
2+
+ 2 e
-
Cu
(s)
, at standard conditions,
for which E° = 0.342 V.
You will vary the silver ion concentration in the test solutions and determine experimentally, the [Ag
+
] at
which the cell potential for reaction (14) is 0.000 V, which is a condition for equilibrium. From this
data, you will calculate the value of K
eq
for reaction (14). You will then compare the experimental value
of K
eq
with the theoretical value of the equilibrium constant obtained from E° values and equation (13).
Part III. The Determination of a Solubility Product
In Part III you will prepare a saturated solution of copper hydroxide (Cu(OH)2)
and determine the
solubility product constant of Cu(OH)2. In making the solution, 1.0 mL of 0.10 M CuSO
4
will be
added to specified volumes of 1.00 M NaOH. The limiting reagent in this reaction will be the Cu
2+
cations. Use of an excess amount of anion allows for a relatively simple calculation of the anion
concentration after reaction, from the stoichiometry of the reaction and your knowledge of the amounts
of reagents mixed together.
Let us assume that most of the copper sulfate reacts. After reaction, the concentration of copper ions
is, therefore, very small (
0). By using Cu
2+
(1.00 M)/Cu as the reference half-cell, you will measure
the cell potential. Then by using the Nernst equation, you will calculate the actual [Cu
2+
] for the
reaction solution, and verify that indeed, the concentration of copper ions in the saturated solution is
extremely low. The concentration of carbonate remaining in solution after reaction is calculated by noting
the stoichiometric relationship between the moles of the limiting reagent, Cu
2+
(aq)
, and the moles of excess
reagent, OH
-
(aq)
. The error in the determined value for the Copper ion concentration will depend on your
error in measuring E
cell
, which will be much larger than your error in measuring the volume of NaOH.
The measurement of E
cell
will therefore determine the error in your value of K
sp
.
Part IV. The Determination of a Formation Constant (also known as a Stability Constant)
You will determine the equilibrium constant for the reaction
Cu
2+
(aq)
+ 4 NH
3(aq)
[Cu(NH3)
4
]
2+
(aq)
(15)