D-11
b)
Calculate the pressure of N
2(g)
using the relation given on the page D-3. Show calculations for all runs.
(0.5 marks)
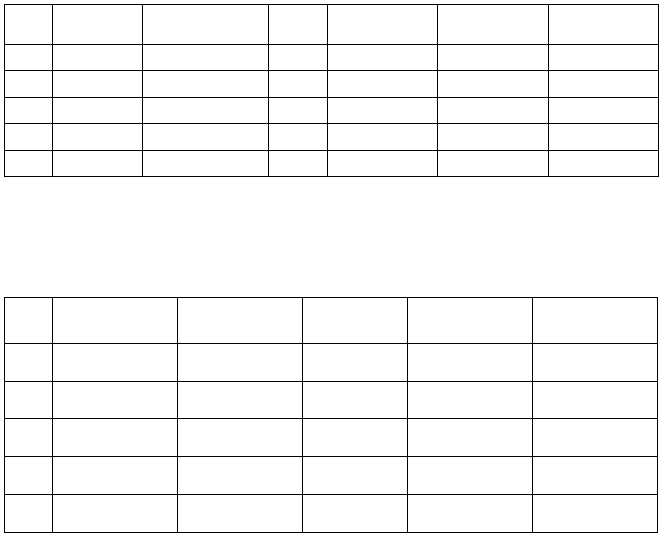
Run
t (°C)
Room temp.
Barometric Pressure
(mm Hg)
h
wc
(mm)
P
wc
(mm Hg)
P
H2O
(g)
(mm Hg)
P
N2
(mm Hg)
1
2
3
4
5
4.
Calculation of R: Convert all of your experimental quantities to the desired units and calculate the value for R
for each run. (Show calculations including units of all runs.) Record your data in the following Table. (1.5
marks).
Run
Volume of N2
(g)
(liters)
Pressure of N2
(g)
(atm)
T (K)
Room temp.
n
(moles of N
2(
g)
)
R
(calculated)
1
2
3
4
5
5.
Calculate the percent error in the average of the experimentally determined R values.
(NOTE: Exclude any inconsistne values from the average.) (0.5 marks)