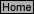30
mass
volume
=
4.126 g
1.62 mL
= 2.603 g mL
-1
The answer with the accepted number of significant figures is 2.60 g/mL
(three significant figures). In multiplication or division, the answer cannot contain more
significant figures than the number that had the least significant figures.
3.
Logarithms
When converting a real number to a logarithmic value, the number of decimal places in the
log value is equal to the number of significant figures in the original number.
Examples:
log 2.33 x 10
6
= 6.367
(three significant figures)
log 2.3 x 10
6
= 6.37
(two significant figures)
The reverse applies when converting from log values to real numbers.
Example:
inverse log 3.777= 5.98 x 10³
(three significant figures)
inverse log 3.77= 6.0 x 10³
(two significant figures)
C.
Average Value
In many experiments you will make two or three determinations of some quantity such as moles or
concentration and will be asked to report the average value. Because the number of observations
is too small to allow for valid statistical interpretations, you should use common sense, especially
if you have some justification for doing so. If you are aware that you have an error in technique,
obviously that measurement should be discarded. If, for example, you make three determinations
and find that two of them agree rather closely while the third is far off (greater than 10%), you
should drop the third and report the average of the two that agree more closely.
There is one other important point to keep in mind. Since many of the techniques that you will be
using are new to you, the value obtained in the last measurement should be better than those
obtained in earlier measurements. This statement simply assumes that “practice makes perfect”
(or at least that we learn by experience), which is a reasonable assumption.
D. Graphing