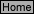
G-7
ADVANCE STUDY ASSIGNMENT
1.
How do the Arrhenius and Brønsted-Lowry definitions of acid and base differ?
2.
Fill in the table
pH
[H3O
+
]
13.134
1.00 x 10
-3
M
3.
You measure the pH of a 0.250 M solution of a weak acid to be 2.36. What is the percent dissociation
of the acid (to the correct number of significant figures)?
Answer: 1.7%
4.
Define the equivalence point for the titration of a monoprotic acid with sodium hydroxide.
0
2
4
6
8
10
12
14
pH
0
10
20
30
40
50
Volume of NaOH added (mL)
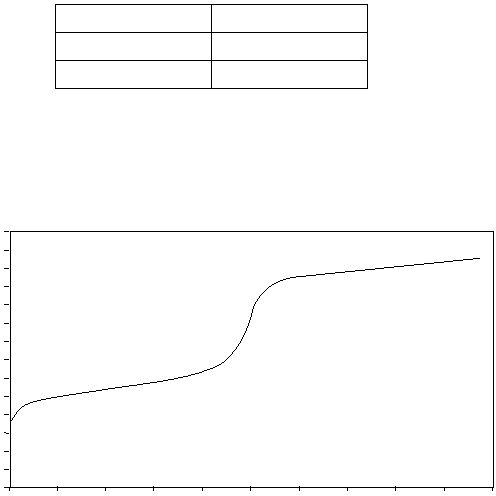
Titration of Unknown Acid using 0.500 M NaOH
5.
Use the titration curve shown above to answer the following questions.
(a) If 50.0 mL of unknown acid was titrated, what was its original concentration?
(b) What is the percent dissociation of this acid before the titration begins?
(c) What are the most significant ions present in solution at the equivalence point?
(d) What is the concentration of the conjugate base at the equivalence point?
(e) What is the pH of the equivalence point?
(f) What is the K
a
of the unknown acid?
6.
What criterion would you use when choosing an acid-base indicator for a given acid? For the example
in question 5, suggest an indicator that might be used for this titration.