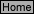G-6
K
in
=
[H3O
+
][In
-
]
[HIn]
The color of an indicator is sensitive to the pH because HIn (acid form) and In
-
(base form) have different
colors. The color of the solution containing the indicator will depend on the relative proportions of the In
-
and
the HIn forms, which will determine the [H3O
+
]:
[H3O
+
] = K
in
x
[HIn]
[In
-
]
If you add a trace (1 or 2 drops) of indicator to your acid solution and then titrate the acid with NaOH solution,
the indicator changes color when the pH of the solution approaches the pK
in
of the indicator. If you have a
good estimate of the pH at the equivalence point for your acid solution, you can choose an appropriate indicator
whose pK
in
is close to this pH and the equivalence point will be accurately indicated by the color change of the
indicator. If the pH at the equivalence point is expected to be near 7, the Table of Indicators on the Data
Sheet shows that bromothymol blue or phenol red would be suitable indicators. If the equivalence point is
expected near pH = 9, thymol blue or phenolphthalein would be suitable. If you were to add phenolphthalein to
a sample of the acid (Figure 2) and titrate it, the end point volume of the titration should match the equivalence
point volume: the change in color from colorless to pink will occur when the equivalence point volume of base
had been added. The concentration of solutions of this acid can therefore be determined by titration using
phenolphthalein as the indicator, without employing a pH meter.
NOTE:
You will be required to plot your data from the pH titration on graph paper as you
perform the titration. Therefore, come prepared to plot the graph during the
laboratory session by labeling, in advance, the axes on a piece of 1.0 mm grid graph
paper in the following manner: pH scale on the y-axis (short axis) from 0-14 and the
volume of NaOH on the x-axis (long axis) from 0 – 48 mL.