G-3
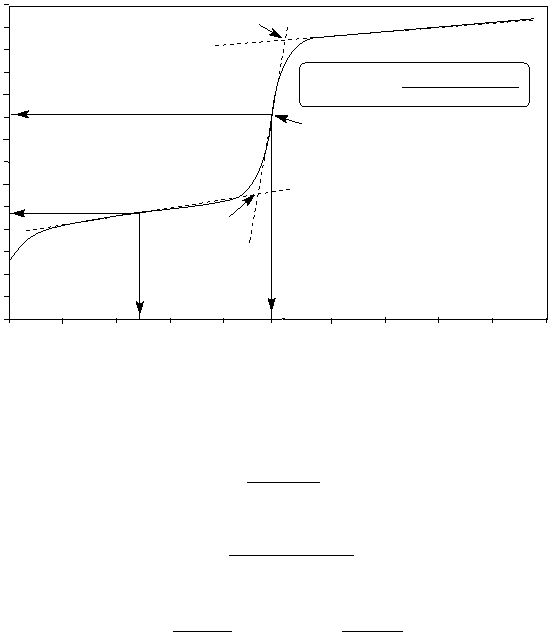
Figure 2
pH at C + pH at D
2
=
pH at Eq. Pt
0
2
4
6
8
10
12
14
pH
0
10
20
30
40
50
Volume of NaOH added (mL)
Titration of HA using 0.50 M NaOH
C
D
Eq. Pt.
pH = 9.06
Eq. Pt.
Eq. vol. = 25.0 mL
pH = pK
a
= 4.72
1/2 Eq. vol.
The extent to which HA dissociates in solution is described by two quantities. The first is the equilibrium
constant for dilute weak acid solutions, called the
acid dissociation
constant, and is represented by the
following relation:
K
a
=
[H3O
+
][A
-
]
[HA]
(4)
Second, the percent dissociation:
% dissociation =
amount dissociated
original amount
x 100%
(5)
For an acid dissolved in pure water the percent dissociation can also be described by
% dissociation =
[A
-
]
i eq
[HA]
initial
x 100% or
[H3O
+
]
i eq
[HA]
initial
x 100%
(6)
where [ ]
i eq
denotes the initial equilibrium concentration, (i.e. before any sodium hydroxide is added), and
[HA]
initial
is the concentration of the weak acid before dissociation occurred. Thus, the percent dissociation of
a weak acid in pure water can be calculated from the initial concentration of acid and the initial pH of the
solution. The percent dissociation is greater for dilute solutions than for concentrated ones.
In Figure 2, the pH value at the start of the titration is about 2.52, so the [H3O
+
] before the addition of any
NaOH is 0.0030 M. (Note: The digits to the right of the decimal in the pH value correspond to the number of