N-5
1) Breaking the O-H bond in the weak acid, an endothermic reaction (?H = positive)
2) Hydration of the H
+
ion and the weak acid anion (CH3COO
-
in the case of acetic acid)
Hydration is the process by which water molecules interact with the ions due to ion-
dipole attraction forces, and is always an exothermic process.
In part three of this experiment you will determine the enthalpy of neutralization of HCl (?H
neut
) and the
enthalpy of neutralization of acetic acid (?H
overall
). From these two experimental results, you will be
able to determine the enthalpy of dissociation of acetic acid (?H
diss
).
EXPERIMENTAL METHOD
The enthalpy changes of chemical reactions are measured in a device called a calorimeter.
Calorimeters are constructed to provide good heat insulation from its surroundings. Under the
conditions of this experiment, the pressure is constant. Hence the heat flow that we will measure is q
p
,
the heat flow at constant pressure.
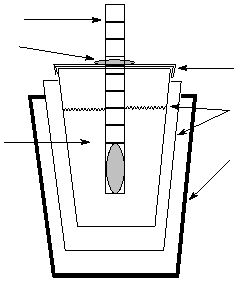
Figure 1:
Polystyrene Calorimeter
Solution
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
Thermometer
Rubber Band
Cover
Polystyrene cups
400 mL Beaker
In this experiment, the calorimeter consists of two polystyrene cups, one nested inside the other, with
the inside cup covered. These are placed in a 400 mL beaker, as shown in Fig. 1. Some textbooks,
including yours, call this a "coffee cup" calorimeter. You will insert your thermometer through a hole in