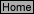T-4
rate =
[
]
I
t
2
= k[S2O
8
2-
][I
-
]
(4)
In this experiment, you will examine the effect of temperature on the rate of reaction (3). Then, using
equation (2) you will obtain the activation energy, E
a
. You will also study the effect of a catalyst on the
activation energy and see how this affects the rate of the reaction.
ADVANCE STUDY ASSIGNMENT
1.
If the equation y = mx + b is plotted graphically:
a. What symbol in the above expression represents the slope of the line?
_________________
b. What is the value of y when x = 0 (sometimes called the y intercept)?
_________________
2.
Compare the equation:
ln k = -
RT
a
E
+ ln A
to the straight-line equation, y = mx + b. Is it necessary to know A to solve for E
a
in this
experiment?
Answer: No, since E
a
is obtained from the slope of the line.
3.
The drawing in Figure 1 shows a reaction in which
H
rxn
is negative and E
a
is positive. Sketch a
drawing that shows potential energy against reaction coordinate for a reaction in which
H
rxn
is
positive and E
a
is positive.
4.
How does the catalyst change the reaction rate? In what way does the catalyst do that?
Answer: A catalyst increases the reaction rate by providing an alternate reaction mechanism that
has a lower E
a
.
5.
What percent of peroxydisulfate (S2O
8
2-
) has been consumed in Run 1 of this experiment when
the blue color appears if 20.0 mL of 0.040 M KI was mixed with 20.0 mL of 0.060 M
(NH
4
)2S2O
8
and 10.0 mL of 7.00 x 10
-4
M Na2S2O3?
Answer: 0.3%.
REAGENTS AND EQUIPMENT
0.04xx M KI (potassium iodide)
0.100 M disodium EDTA
0.06xx M (NH
4
)2S2O
8
(ammonium peroxydisulfate)
3% starch solution
7.xx x 10
-4
M Na2S2O3 (sodium thiosulfate)
automatic dispenser for Na2S2O3
0.060 M (NH
4
)2SO
4
(ammonium sulfate)
0.10 M CuSO
4
(copper(II) sulfate)