T-17
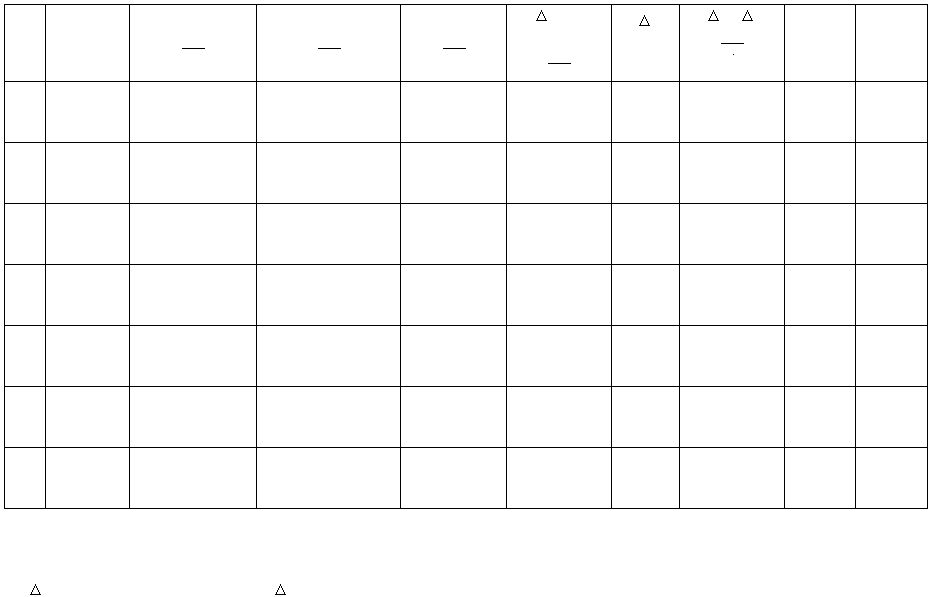
Table I. The Effect of Temperature on the Reaction Rate of KI and (NH
4
)2S2O
8
.
Run
Total
Reaction
Volume
[KI]*
mol
L
[(NH
4
)2S2O
8
]*
mol
L
[S2O3²
-
]*
mol
L
[I2]**
Formed
mol
L
t
sec
[I2]/
t
mol
L
s
k
Temp
°C
1.
2.
3.
4.
5.
6.
7.
*The molarity recorded should be the molarity in the solution, after mixing but before reaction, not the molarity of the reagents in the stock
solution.
**
[I2] is the amount of [I2] formed during
t, that is, before the blue color appears.