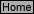S-11
6
6.67
13.33
5 drops
2 drops
10.00
20.00
-
2 drops
7
10.00
10.00
5 drops
2 drops
10.00
10.00
10.00
2 drops
The 125 mL Erlenmeyer flasks and 100 mL beakers must be cleaned thoroughly before use. Make
sure that there are no traces of soap remaining in the beaker or flask. Before each run, rinse the
glassware four times with tap water and twice with DI water
then DRY the glassware so the
reagent solutions are not diluted.
Carefully measure the solutions into the 125 mL Erlenmeyer flask as outlined in Table I. Similarly,
measure the solutions into the 100 mL beaker.
NOTE:
The volumes used do not need to be a specific preset amount. They need only to be
close, but they must be accurately measured (i.e. to 0.02 mL).
Remember to add 2 drops of EDTA* to each container. All solutions are at room temperature.
Record the temperature of solution in the flask as the temperature of the reaction solution.
Have your timer ready and quickly pour the solution from the beaker into the flask and swirl the
solution gently to promote mixing. Continue to swirl the solution in the flask until the first trace of blue
appears. The time for the reaction should be taken from the point of mixing
(i.e. t = 0) to the first appearance of the blue color. Immediately record the time and then the
temperature of the reaction solution in the Table on the Observations Sheet.
Repeat the above run with a second set of samples (Run 2, Table I) for a second determination of the
reaction rate at these concentrations.
Using the same procedure as outlined above, carry out rate measurements for the remaining 5 solutions
described in Table I. If the data appear unreasonable, you may wish to perform duplicate runs.
Remember, the more data you have the more accurate your results will be. In each experiment, the
volume of KI and (NH
4
)2S2O
8
should be measured accurately from the burets at your work station.
The volumes of KCl and (NH
4
)2SO
4
can be measured from the burets set up in the laboratory for
common use. The latter two solutions are added to keep the volume of the reaction mixture constant
(i.e. keep the ionic strength constant). They do not participate in the reaction.
*
Traces of copper from previous experiments may catalyze this reaction. EDTA is added to the solutions to complex
with any stray metal ions that might be present.