S-10
0.060 M (NH
4
)2SO
4
(ammonium sulfate)
EXPERIMENTAL PROCEDURE
I.
Preparation of Solutions.
NOTE: In order to reduce the time to set up for and cleanup after this experiment, two
students may share one pair of reagent burets.
a)
Set up 1 pair of burets to dispense these reagents for two students. Using the correct
technique, rinse and fill one buret with the (NH
4
)2S2O
8
solution. Record the concentration of
this solution on your Observations Sheet. Rinse and fill the second buret with the KI
solution. Record this concentration on your Observations Sheet.
NOTE: This experiment is NOT done in pairs. You are to share these burets only.
b)
The standardized Na2S2O3 is dispensed from a bottle with an automatic dispenser. Record
the exact concentration of this Na2S2O3 solution on your Observations Sheet.
II.
Establishing the Rate Law.
The volumes of reagents needed for each run are given in Table I. Use the burets at your work
station to dispense the (NH
4
)2S2O
8(aq)
and the KI
(aq)
solutions. The (NH
4
)2SO
4(aq)
or KCl
(aq)
solutions are to be dispensed as required from burets set up in the laboratory for general use.
Again, the Na2S2O
3(aq)
will be dispensed in 10.00 mL portions using the automatic dispenser.
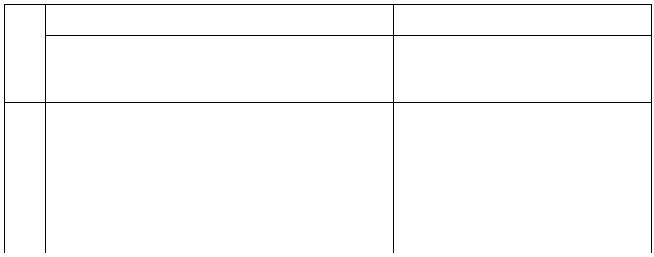
Table I. Volumes of Reagents (mL)
125 mL Erlenmeyer Flask (V
TOTAL
~ 30.0 mL)
100 mL Beaker (V
TOTAL
~ 20.0 mL)
Run
#
KI
(mL)
KCl
(mL)
Starch
EDTA
Na2 S2O3
(mL)
(NH
4
)2S2O
8
(mL)
(NH
4
)2SO
4
(mL)
EDTA
1
20.00
5 drops
2 drops
10.00
20.00
-
2 drops
2
20.00
5 drops
2 drops
10.00
20.00
-
2 drops
3
20.00
5 drops
2 drops
10.00
10.00
10.00
2 drops
4
20.00
5 drops
2 drops
10.00
6.67
13.33
2 drops
5
10.00
10.00
5 drops
2 drops
10.00
20.00
-
2 drops