Q-3
reactions. An easier, and more quantitative, way of ranking the reduction potentials of reagents can be
achieved by measuring the voltage (potential difference) in a Voltaic Cell (also called Galvanic Cell) such
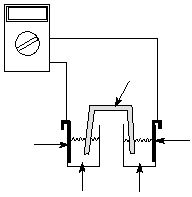
as that shown in Figure 1.
Salt Bridge
1.100 V
Voltmeter
Zn(NO
3
)
2
(1.0 M)
CuSO
4
(1.0 M)
Zinc Metal
Copper Metal
The reactants are in separate compartments, and the circuit is completed by a salt bridge (filter paper
wetted with KNO3 in this experiment) and an external wire connection through a voltmeter. The salt
bridge maintains ion charge neutrality in each half-cell and allows a current to flow, but prevents physical
mixing of the reagents in the two half-cells. Ideally, the voltmeter should draw no current. Electrons flow
from the Zn anode, which is the negative electrode, to the Cu cathode, which is the positive electrode,
through the connecting wire. Reduction always occurs at the cathode and oxidation always occurs at the
anode.
When the positive terminal of the voltmeter is connected to the cathode, and the negative terminal is
connected to the anode, a positive voltage is measured on the voltmeter. The magnitude of this voltage,
called the cell voltage, is a measure of the driving force, or the degree of spontaneity of the reaction. For
the cell shown in Fig. 1, the measured voltage is about 1.10 volts. If the 1.0 M CuSO
4
solution was
replaced by 1.0 M H2SO
4
, reduction of H
+
(aq)
would replace the reduction of Cu
2+
(aq)
, and the measured
voltage would be about 0.76 volts. These observations give both the relative ordering of the reducing
agents as Zn > H2 > Cu, and a quantitative measure of that ordering.
Figure1