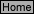Q-2
therefore, Zn
(s)
must have a greater tendency to lose electrons than H
2(g)
. However, if we were to replace
Zn
(s)
with Cu
(s)
in reaction (1), we would find that Cu
(s)
does not react with H
+
(aq)
to give H
2(g)
and Cu
2+
(aq)
.
Therefore Cu
(s)
is a weaker reducing agent than H
2(g)
, or stated the other way around; H
2(g)
must be a
stronger reducing agent than Cu
(s)
.
When ranking the oxidizing-reducing power of reagents, it is conventional to write them in terms of
hypothetical electrochemical half-reactions, with the reduced form of the reagent on the right-hand
(product) side. The stronger reducing agents (see Chemistry Data Sheet) are usually written at the top of a
table of such half reaction equations.
Following this convention, we would summarize the results of the previous paragraph as follows:
Zn
2+
(aq)
+ 2 e
-
Zn
(s)
(stronger reducing agent)
(2)
2 H
+
(aq)
+ 2 e
-
H
2(g)
(3)
Cu
2+
(aq)
+ 2 e
-
Cu
(s)
(weaker reducing agent)
(4)
With this kind of ordering, an equation for a spontaneous reaction can be found by taking a reduction
half-reaction equation and adding to it the any oxidation half-reaction equation (i.e. the reverse of
the reduction reaction) above it in the Table. Thus, the reactions obtained by
(eq 4 plus the reverse of eq 2) giving:
Cu
2+
(aq)
+ Zn
(s)
Cu
(s)
+ Zn
2+
(aq)
(5)
and
(eq 4 plus the reverse of eq 3) giving:
Cu
2+
(aq)
+ H
2(g)
Cu
(s)
+ 2 H
+
(aq)
(6)
are spontaneous as written. In the previous experiment, using simple qualitative chemical tests, this same
ordering was discovered. Using similar comparisons you will determine the ordering for the reduction
potentials of the halogens in this experiment.
The relative ordering of redox half-cells can be accomplished using a procedure that is similar to that
described in Experiment P, however, it would require the pairing of every possible permutation of half-cell