M-5
In this equation,
is the molar absorptivity (units M
-1
cm
-1
), a constant characteristic of a
particular chemical species at a particular wavelength; b is the path length or distance photons
must travel (in centimeters) through the solution; and c is the concentration of the absorbing
species in solution. The term A represents absorbance which is a dimensionless quantity that
expresses how much light is being absorbed by the sample (see below).
There are two scales that can be used to measure the interaction of electromagnetic radiation
with an absorbing species, specifically transmittance and absorbance. Transmittance (T) is
defined as the fraction of the original light that passes through the sample. It is simply a measure
of the light energy that is transmitted through a sample and is expressed as a ratio of the light
transmitted through the sample (I) divided by the incident (in-coming) light intensity (I
o
)
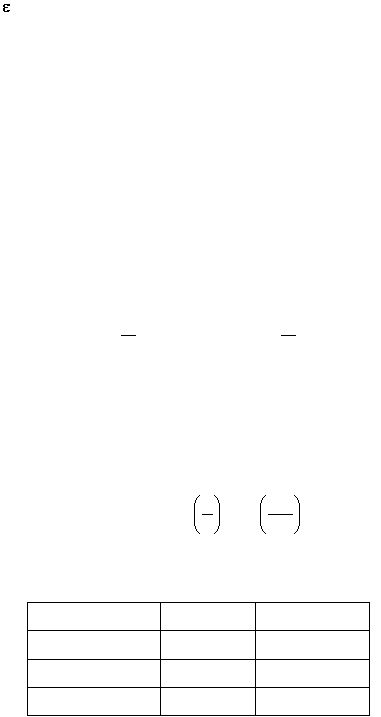
T =
o
I
I
and
%T =
o
I
I
x 100%
Percent transmittance is a linear scale that extends from 0 %, which corresponds to all the light
being absorbed, to 100 % which corresponds to none of the light being absorbed.
A more useful relationship is absorbance (A) which is a logarithmic expression of transmittance.
A = log
T
1
= log
T
%
100
The following table shows the relationship between these two scales.
T = I/I
o
% T
A
1.0
100
0
0.1
10
1
0
0
infinity
The Beer Lambert law relates the absorbance,
A, not transmittance, of a solution to the
concentration of the light absorbing species (c), in solution.
Beer’s Law may be used to quantitatively determine the concentration of the light absorbing
species by selecting a specific wavelength and pathlength to measure absorbance. Under these
conditions, absorbance will vary linearly with the concentration of the absorbing species in