M-16
Part III: Calibration Curve
1.
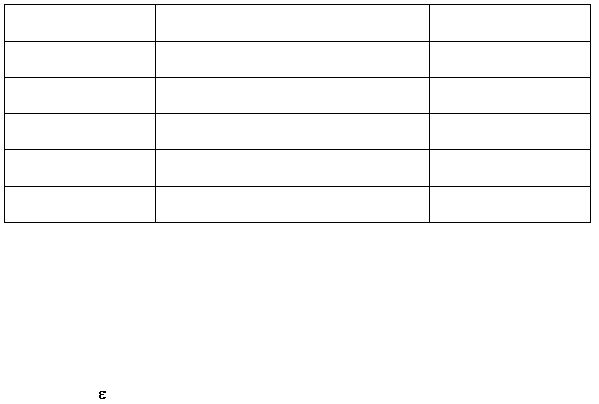
Complete the following table correlating absorbance to the concentration of the
[Fe(Bipy)3]
2+
complex: (0.2 marks)
Standard Solution
Concentration of Fe
2+
Absorbance, A
0.00
0.00
1.
2.
3.
4
2.
Prepare a calibration curve for the [Fe(Bipy)3]
2+
complex by plotting the above data in a
graph of absorbance versus concentration. (Again, use 1.0 mm grid paper labeled
clearly). (0.6 marks)
3.
Calculate the slope of the calibration curve. Using this slope value, determine the molar
absorptivity,
, for the [Fe(Bipy)3]
2+
complex. (Assume the path length of the cuvette is
1.20 cm.) (1.0 mark)
4.
Explain why the pH of the of [Fe(Bipy)3]
2+
complex solution needs to be kept close to
pH 4.5? (1.0 mark)