G-4
The extent to which HA dissociates in solution is described by two quantities. The first is the equilibrium
constant for dilute weak acid solutions, called the
acid dissociation
constant, and is represented by the
following relation:
K
a
=
[H3O
+
][A
-
]
[HA]
(4)
Second, the percent dissociation:
% dissociation =
amount dissociated
original amount
x 100%
(5)
For an acid dissolved in pure water the percent dissociation can also be described by
% dissociation =
[A
-
]
eq
[HA]
initial
x 100% or
[H3O
+
]
eq
[HA]
initial
x 100%
(6)
where [ ]
eq
denotes the concentration at equilibrium and [HA]
initial
is the concentration of acid before
dissociation occurred. Thus, the percent dissociation of an acid in pure water can be calculated from the initial
concentration of acid and the pH of the solution. The percent dissociation is greater for dilute solutions than for
concentrated ones.
In Figure 2, the pH value at the start of the titration is about 2.52, so the [H3O
+
] before the addition of any
NaOH is 0.0030 M. (Note: The digits to the right of the decimal in the pH value correspond to the number of
significant digits in the calculated value of [H3O
+
]). Since
[HA]
initial
= 0.50 M, therefore, only 0.60% of HA is dissociated into hydronium ions and A
-
in the initial
solution.
The equivalence point of the titration is the point in a titration where the moles of titrant added are
stoichiometrically equivalent to the moles of substance being titrated, i.e. the analyte. For a weak acid, the pH
of the equivalence point does not necessarily occur at pH = 7. The pH of the equivalence point is dependent
on the K
a
of the acid being titrated and on its initial concentration.
The equivalence point must be determined exactly in order to find the K
a
for the weak acid, as well as to select
a suitable indicator for titrations using acid-base indicators. The equivalence point for the titration can be
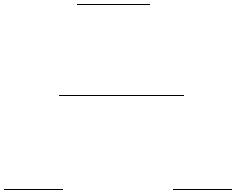
determined from the pH titration curve. This is done by first drawing an extended straight line through both of
the nearly horizontal regions of the graph before and after the equivalence point. Next, a tangential line (C-D)
is drawn through the actual data points in the vertical portion of the titration curve to intersect the previous two