F-3
solution and the amount of I
-
that reacted with I2 to form I3
-
. The amount of I
-
that reacted is
stoichiometrically equivalent to the amount of I3
-
formed (i.e. the [I3
-
] from equation (6)):
[I
-
(aq)
] = [I
-
]
(initial)
- [I3
-
(aq)
]
(7)
This relationship applies because of the 1:1 stoichiometric ratio between the I
-
(aq)
reacted and the I3
-
(aq)
formed.
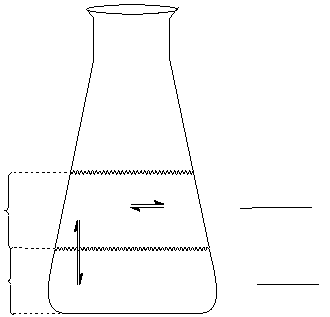
A careful study of Figure 1 will help you understand the various equilibria involved. K' has been given to
you in equation (4), so that you will be able to determine K experimentally. Note that there is direct
competition between the two equilibria. This competition can be illustrated by considering the following
example. Let us assume that [I
2(CH2Cl2)
] is decreased somehow:
1.
Then equilibrium (3) would be disturbed (the value of Q' becomes less than K').
2.
Because the value of Q' is less than K', I
2(aq)
is drawn from water into CH2Cl2 to
re-establish equilibrium (3).
3.
However, this decrease in [I2] disturbs equilibrium (1). To re-establish equilibrium (1), some I3
-
(aq)
dissociates to form I
-
(aq)
and I
2(aq)
.
The competition between these equilibria is another example of interactive equilibria that you studied
in Experiment E.
=
1.50 x 10 ²
(Purple)
Figure 1
CH 2Cl 2
layer
aqueous
layer
[I
2(aq)
]
[I
2(CH
2
Cl
2
)
]
K'
=
K
eq
K'
+
I
2(CH
2
Cl
2
)
I
3(aq)
-
I
(aq)
-
I
2(aq)
[I
2(aq)
]
-
[I
(aq)
]
-
[I
3(aq)
]
K
eq
=