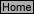E-7
Fe
3+
(aq)
+ 3 OH
-
(aq)
Fe(OH)
3(s)
Fe
3+
(aq)
+ 5 F
-
(aq)
+ H2O
(l)
[FeF
5
(H2O)]²
-
(aq)
Fe
3+
(aq)
+ 3 C2O
4
2-
(aq)
[Fe(C2O
4
)3]³
-
(aq)
4 Fe
3+
(aq)
+ 3 [Fe(CN)
6
]
4-
(aq)
Fe
4
[Fe(CN)
6
]
3(aq)
Fe
3+
(aq)
+ 3 NH
3(aq)
+ 3 H2O
(l)
Fe(OH)
3(s)
+ 3 NH
4
+
(aq)
Ag
+
(aq)
+ NCS
-
(aq)
AgNCS
(s)
While you are performing the experiment, keep in mind that Fe(OH)
3(s)
is rust coloured, AgNCS
(s)
is white,
Fe
4
[Fe(CN)
6
]
3(aq)
is very dark blue, and both [Fe(C2O
4
)3]³
-
(aq)
and [FeF
5
(H2O)]²
-
(aq)
are colourless .
In Part II, you will examine the solubility of several lead compounds: lead nitrate (Pb(NO3)2), lead chloride
(PbCl2), lead chromate (PbCrO
4
), and lead sulfide (PbS). You will be given a solution of Pb(NO3)2. From this
you will prepare PbCl
2(s)
, which is only slightly soluble in water. The solubility equilibrium for the saturated
solution formed is:
PbCl
2(s)
Pb
2+
(aq)
+ 2 Cl
-
(aq)
(9)
If some chloride ions are added to the saturated solution of PbCl2, you would expect the position of equilibrium
to shift to the left, precipitating more solid. However, at very high concentrations of chloride ions, another
reaction becomes significant: Pb
2+
(aq)
reacts with Cl
-
(aq)
to form a water-soluble complex ion, [PbCl3]
-
(aq)
:
Pb
2+
(aq)
+ 3 Cl
-
(aq)
[PbCl3]
-
(aq)
(10)
In this experiment, you will examine the reactions of lead in both low and high concentrations of chloride.
To establish the relative solubilities of PbCl
2(s)
, PbCrO
4 s)
and PbS
(s)
, you will first add sequentially, aqueous
solutions of sodium chromate (Na2CrO
4
) and sodium sulfide (Na2S) to a test tube containing some solid PbCl2
in equilibrium with its dissolved salts. Following this you will look at the reverse reactions by adding
Na2CrO
4(aq)
and NaCl
(aq)
to a solution containing solid PbS.
From your observations, you will be able to rank
these three sparingly soluble salts in the order of decreasing solubility. It will help you to know that the PbCl
2(s)
is white, PbCrO
4(s)
is yellow, and PbS
(s)
is black.