E-14
OBSERVATIONS & INFORMATION SHEET - EXPERIMENT E
Le Châtelier's Principle and Interactive Chemical Equilibrium
Part I. The Iron(III) Thiocyanate Equilibrium
Write the balanced net ionic equation for the equilibrium reaction between Fe(NO3)3
(aq)
and KNCS
(aq)
.
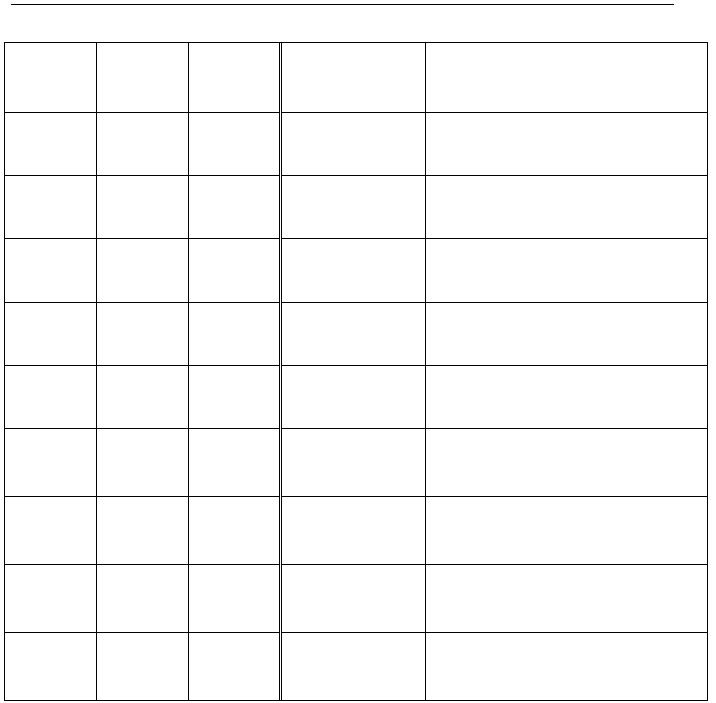
Observations for the addition of various reagents to samples of the above equilibrium solution.
reagent(s)
added
description
of reactants
description of reaction
solution once
equilibrium is
re-established
i.
interactive equilibria product(s) formed
ii.
the effect on the concentration of
[Fe(NCS)]
2+
(aq)
Test Tube 1
5 drops H2O
colourless
liquid
medium rust-red
solution
i.
none
ii.
slight dilution of [Fe(NCS)]
2+
(aq)
***CONTROL - reference colour
Test Tube 2
i.
______________________________________
ii.
______________________________________
Test Tube 3
i.
______________________________________
ii.
______________________________________
Test Tube 4
i.
______________________________________
ii.
______________________________________
Test Tube 5
i.
______________________________________
ii.
______________________________________
Test Tube 6
i.
______________________________________
ii.
______________________________________
Test Tube 7
i.
______________________________________
ii.
______________________________________
Test Tube 8
i.
______________________________________
ii.
______________________________________
Test Tube 9
i.
______________________________________
ii.
______________________________________
___________________________
_____________________________
_______________
Signature of Laboratory Instructor
Name of Student
Laboratory Section