April 23, 2001
CHEMISTRY 105(400)
Instructor: Dusan Ristic-Petrovic
FINAL EXAMINATION
Weight: 35%
Name: ________________________ I.D. # _________________
Signature: ______________________
Time for writing: 3 hours
READ ALL OF THE QUESTIONS CAREFULLY.
SHOW ALL OF YOUR REASONING TO OBTAIN FULL MARKS
Question Value Score
1. 5 _____
2. 7 _____
3. 8 _____
4. 4 _____
5. 5 _____
6. 8 _____
7. 7 _____
8. 12 _____
9. 4 _____
10. 7 _____
11. 6 _____
12. 6 _____
13. 6 _____
14. 5 _____
Total 90 _______
Bonus (2) _______
[5] 1. For the combustion reaction
CH4(g) + 2O2(g) ¾¾® CO2(g) + 2H2O(l)
a) Calculate DGo and DHo at 298K
b) How much heat would be evolved if:
i) No useful work was done by the combustion reaction?
ii) The maximum amount of useful work was done?
[7] 2. The activation energy for the decomposition of ammonia gas into N2 gas and H2 gas is decreased from 350 to 162 kJ/mol in the presence of a tungsten catalyst.
a) By what factor will the reaction rate be increased by the tungsten catalyst
at 700.oC?
b) According to collision theory, what does the capital “A” in the Arrhenius
equation represent?
c) Which of the following changes will affect the numerical value for the rate constant kobs? Circle your choice(s).
i) an increase in temperature
ii) a doubling of the reactant concentration
iii) the addition of a catalyst
iv) changing the solvent from water to an organic solvent
[8]. 3. Provide a sketch for the galvanic cell
Sn/Sn2+(aq)(1.00 M)//Cr2O72-(aq) (1.00 M), H+(aq)(1.00 M), Cr3+(aq) (1.00 M)/Pt
a) Give the overall cell reaction, identify the anode and the cathode and calculate the cell voltage at standard conditions.
Question # 3 Continued
b) Calculate DGo and Keq for the cell reaction at 25oC.
c) Calculate the cell potential at 25oC if the concentrations of all of the
dissolved metal ions are kept the same but the pH in the cathodic
compartment is increased from 0.000 to 3.000.
[4]
4. a) Given the following standard reduction
potentials, show that UO2+
will spontaneously
disproportionate in acidic solution at standard
conditions:
![]()
b)
Is UO2+ a better oxidizing agent or reducing
agent? Justify your answer.
[5] 5. The standard enthalpy of formation for HCN(g) (i.e DHof HCN(g)) is
135.1 kJ/mol. By using this piece of information along with the appropriate
thermodynamic data from the data sheet, calculate the bond energy for the CN
bond in HCN. (Note: Each HCN molecule is held together by one CN bond
and one CH bond, i.e. the connectivity is H-C-N)
[8] 6. The half-life for the second order decomposition…
![]()
is 32.0 minutes when the initial concentration of A is 0.280 M.
a) Write down the differential rate law for the reaction.
b) How many minutes would it take for the concentration of A to drop
from 0.280 M to 0.0122 M?
c) Calculate the concentration for both A and B at t = 200.0 minutes.
d) Estimate the instantaneous rate of reaction at t = 200.0 minutes.
[7] 7. Molecular chlorine can be prepared by reacting molecular oxygen with
hydrogen chloride gas:
4HCl(g) + O2(g) ¾¾® 2Cl2(g) + 2H2O(g)
The Kp for this reaction is 1.55 x 103 atm-1 at 400 K and
5.25 x 10-5 atm-1 at 800 K.
a) Find the DHo for the
reaction without using D
Question # 7 Continued
b) Calculate DGo and Kp for the forward reaction at 25oC.
c) Determine the direction of the spontaneous reaction when:
PH2O = 1.00 atm, PCl2 = 1.00 atm
PO2 = 1.00 x 10-3 atm, PHCl = 1.00 x 10-3 atm and T = 298 K.
[12] 8. Complete the following table:
Molecular Lewis Dot Sketch of the VSEPR Molecular Is the
Species Structure Molecule (with Notation Shape molecule
lone pairs) polar?
SeF4
SbCl5
PF6-
[4] 9. a) Draw three valid resonance structures for fulminic acid.
Be sure to include all non-zero formal charges. Pick the structure(s)
that most accurately depict(s) the bonding in the molecule. (Note:
The sequence of atoms in the molecule is CNOH).
[7] 10. The following kinetics data were obtained for
the reaction…
![]()
where Reaction Rate = - D[I-]/Dt
[I-], M [Fe3+], M Initial Rate (moles/L.sec)
0.050 0.100 5.75 x 10-2
0.100 0.050 0.115
0.050 0.300 0.1725
a)
Determine the differential rate law and the
value for k.
Question # 10
Continued
b)
Is your empirical rate law consistent with the
proposed mechanism shown
below? Be sure to justify your answer.
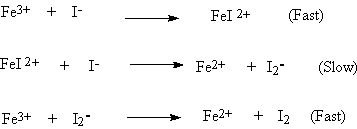
[6] 11. Complete the following short answer questions:
a) The reaction between 1.0 M MnO4-(aq) and 1.0 M Fe2+(aq)1.0 M H+(aq) at 25oC
(spontaneous/non-spontaneous) _____________________. In this reaction, the Fe2+ ion
is acting as the (oxidizing agent/reducing agent) ______________________.
b) Select a reagent from the table of standard reduction potentials
that is capable of oxidizing water and write the balanced equation for the overall
reaction.
c) Can air (0.21 atm O2) oxidize copper metal to Cu2+(aq) at 25oC in a neutral
solution that is 0.10 M in Cu2+(aq)? Justify your answer.
[6] 12. Fill in each blank with the appropriate answer.
a) Lewis theory can be used to explain why boron trifluoride reacts vigourously with ammonia. The Lewis acidity of boron trifluoride is caused by it being ____________________
b) Consider the compounds: LiCl, MgCl2, NaCl, CCl4
Which compound has the greatest ionic character? ______________.
Which compound has the weakest intermolecular bonding? _____________.
c) What intermolecular forces must be overcome to melt
diamond?_____________________________________.
d) Molecular nitrogen (N2) and carbon monoxide (CO) are isoelectronic and have virtually identical molar masses. Which substance has the higher
melting point? ________________.
e) What type of solid (ionic, molecular or network covalent) is
ZrBr4? ______________________ Note: ZrBr4 melts at 105oC and the pure
liquid does not conduct electricity.
[6] 13. The empty molecular orbital diagram for CO is given below. Complete
the molecular orbital diagram and then answer the questions posed below.
Be sure to identify Atoms A and B and don’t forget to label all of the
molecular orbitals in the centre of the diagram.
i) What is the bond order for CO in the ground state?
ii) What is the total number of p-bonds in CO?
iii) Classify CO as either paramagnetic or diamagnetic. Justify your answer.
Atom A Atom B
[5] 14. A researcher requires 10.0 mg of the radioactive isotope 32P, which has a
half-life of 14.3 days. It is supplied as solid Na332PO4.
a) What mass of Na332PO4 should be ordered if the delivery time from the
supplier is 72 hours?
b) What is the initial radioactivity (i.e. the number of atoms that disintegrate
each second) for a 10.0 mg sample of 32P?
Bonus (2 Marks): The standard reduction potential for the conversion of sulfate ions
to sulfur dioxide in acidic solution is 0.160 V:
SO42-(aq) +
4H+(aq) ![]() SO2(aq)
+ 2H2O(l) Eo = 0.160 V.
SO2(aq)
+ 2H2O(l) Eo = 0.160 V.
Use this information and the appropriate equation(s)/reaction(s) from the data
sheet to show that SO2 gas at standard conditions will be spontaneously
converted to sulfuric acid upon contact with any airbourne water droplets.