F-3
competition between the two equilibria. This competition can be illustrated by considering the following
example. Let us assume that [I
2(CH2Cl2)
] is decreased somehow:
1.
Then equilibrium (3) would be disturbed (the value of Q' becomes less than K').
2.
Because the value of Q' is less than K', I
2(aq)
is drawn from water into CH2Cl2 to
re-establish equilibrium (3).
3.
However, this decrease in [I2] disturbs equilibrium (1). To re-establish equilibrium (1), some I3
-
(aq)
dissociates to form I
-
(aq)
and I
2(aq)
.
The competition between these equilibria is another example of interactive equilibria that you studied
in Experiment E.
EXPERIMENTAL METHOD
Chemists are often required to determine ( or to verify ) the amount of a substance (S) in a sample. If S
reacts with a reagent R according to the following hypothetical the reaction:
aS + bR
products
then the amount of S in the sample can be determined by adding R to a stoichiometrically equivalent
amount. This procedure of adding R until it is in a stoichiometrically equivalent amount to S is known as a
titration. The amount of R used is controlled by adding the solution of R from a buret. In a titration, the
equivalence or stoichiometric point is the point at which the moles of substance added, the titrant, is
stoichiometrically equivalent to the number of moles of substance being titrated, the analyte. This point is
usually made apparent by the use of some type of colored indicator which gives an endpoint
=
1.50 x 10 ²
(Purple)
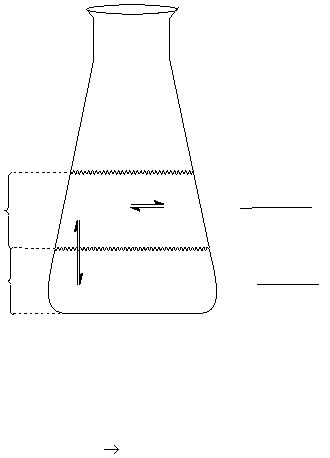
Figure 1
CH 2Cl 2
layer
aqueous
layer
[I
2(aq)
]
[I
2(CH
2
Cl
2
)
]
K'
=
K
eq
K'
+
I
2(CH
2
Cl
2
)
I
3(aq)
-
I
(aq)
-
I
2(aq)
[I
2(aq)
]
-
[I
(aq)
]
-
[I
3(aq)
]
K
eq
=