F-13
Report: Experiment F - Iodine-Triiodide Equilibrium Constant
Name ____________________________________
Section ___________ Date ___________________
Laboratory Instructor________________________
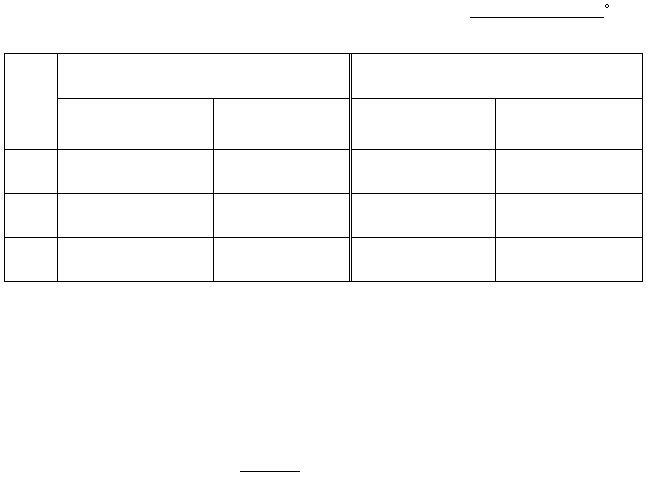
SUMMARY OF DATA:
Initial concentration of I
-
(aq)
(from KI3 reagent bottle)
___________________M
Molarity of S2O3²
-
(aq)
______________M
Room Temperature
C
Part II: Titration of aqueous layer
Part III: Titration of CH2Cl2 layer
Vol. of aqueous layer
(mL)
Vol. of S2O3²
-
(aq)
(mL)
Vol. of CH2Cl2 layer
(mL)
Vol. of S2O3²
-
(aq)
(mL)
Run 1
Run 2
Run 3
All calculations must include stoichiometric ratios, units, and have the correct number of significant
figures. (Do not use C1V1 = C2V2. This relationship applies only to dilution calculations, not to titration
calculations.) Also, do not average your data. An average of the appropriate concentrations will be
found once the titration calculations are completed.
1.
Show your calculations of ([I3
-
+ I2]
(aq)
) at equilibrium in the aqueous layer.
NOTE: The titration is for the combined I
2(aq)
+ I3
-
(aq)
in the aqueous aliquot. (1.0 mark)
Run 1
______________
Run 2
______________
Run 3
______________
average
_____________