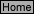E-8
water molecules bonded to the cobalt ion are replaced by chloride ions to form [CoCl
4
]²
-
(aq)
, which is deep blue
in colour. A solution containing roughly equal concentrations of both complex ions is violet (mixture of pink
and blue colours). From the colour of the solution, you will determine which complex ion has the higher
concentration. You will investigate the effect of temperature on this reaction (11). Temperature or heat does
not appear in equation (11), but changing the temperature affects the position of equilibrium. You will be able
to account for the effect of temperature in terms of Le Châtelier's Principle by adding "heat" as a reactant or
product to equation (11), depending on whether the reaction is endothermic or exothermic. The stress created
by the addition (or removal) of heat is relieved by a shift in the equilibrium position of reaction (11).
ADVANCE STUDY ASSIGNMENT
1.
For the arbitrary chemical reaction A + B
2 C, K has a value of 1. You prepare a solution that has
the initial concentrations:
a)
[A] = 1 M, [B] = 1 M, [C] = 2 M. What is the value of the reaction quotient and how would the
concentrations of the reactants and products change (increase or decrease) in order to re-establish
equilibrium?
Answer:
Q = 4 and is greater than K (which equals 1), so the equilibrium shifts to the left. The
reverse reaction occurs and the concentrations of products decrease and the
concentrations of reactants increase.
b)
[A] = 2 M, [B] = 1 M and [C] = 1 M. What is the value of the reaction quotient and how would
the concentrations change in order to reach equilibrium? Explain.
2.
Write net ionic equations for the following reactions in aqueous solutions:
a) Pb(NO3)
2(aq)
and NaCl
(aq)
to form PbCl
2(s)
b) PbCl
2(aq)
and Na2CrO
4(aq)
to form PbCrO
4(s)
c) PbCrO
4(aq)
and Na2S
(aq)
to form PbS
(s)
3.
In which direction does the oxalic acid dissociation equilibrium (equation (7)) shift when H3O
+
(aq)
is
added to a 0.10 M solution of oxalic acid (H2C2O
4
)? Why?
Answer: Shifts to the left, restoring K' by increasing [H2C2O
4
] and decreasing [H3O
+
] and [C2O
4
2-
] .
4.
Predict what happens to the position of equilibrium when OH
-
(aq)
is added to a solution containing a
Fe
3+
(aq),
NCS
-
(aq)
and
[Fe(NCS)]
2+
(aq)
at equilibrium (equation (8))? Why?
Answer:
The added OH
-
(aq)
will react with the Fe
3+
(aq)
causing the [Fe
3+
] to decrease. The
[Fe(NCS)]
2+
(aq)
equilibrium (equation 8) will shift to the left, restoring K by replacing the