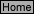E-5
Now, suppose some acetate (CH3COO
-
) is added to the solution. The value of the reaction quotient, Q, for
acetic acid now exceeds the value of the equilibrium constant, K
a
, therefore the system shifts to restore the
equilibrium. Le Châtelier's Principle will also predict that the added acetate will cause a shift in the equilibrium
position of the acetic acid system in order to restore the value of Q to the K
a
value for acetic acid.
Therefore, the net effect of adding acetate ions to the acetic acid-oxalic acid system at equilibrium is to lower
the [H3O
+
]. Lowering [H3O
+
] will in turn make the value of Q for the oxalic acid system less than its K' value
(see equation (7)). Consequently, a shift in the equilibrium position of reaction (7) must occur in order to
restore the K' for oxalic acid. Thus, the oxalate ion concentration, [C2O
4
2-
], will increase and oxalic acid
concentration, [H2C2O
4
], will decrease to restore the value of Q for the oxalic acid system to the K' value. The
net effect is to alter the concentrations of all species in such a way, that both the K
a
for acetic acid and the K' for
oxalic acid are again satisfied.
EXPERIMENTAL METHOD
In Part I, you will examine the equilibrium shown below for the reaction between iron(III) (Fe
3+
) and
thiocyanate (NCS
-
) ions forming the iron(III) thiocyanate complex, ([Fe(NCS)]
2+
):
Fe
3+
(aq)
+ NCS
-
(aq)
[Fe(NCS)]
2+
(aq).
(8)
The equilibrium position for reaction (8) can be shifted to the right by adding more Fe
3+
(aq)
or
NCS
-
(aq)
. Conversely, the equilibrium can be shifted to the left by removing either Fe
3+
(aq)
or NCS
-
(aq)
from
solution. The concentrations of Fe
3+
(aq)
or NCS
-
(aq)
can be decreased by the addition of various reagents that
react with the either the free Fe
3+
(aq)
or NCS
-
(aq)
to form complex ions. For example, the hydroxide anion reacts
with free Fe
3+
(aq)
to form insoluble Fe(OH)
3(s)
. The equilibrium system described by reaction (8) responds to a
drop in [Fe
3+
] by shifting to the reactant side replacing the depleted Fe
3+
(aq)
until equilibrium is re-established.
Similarly, the [NCS
-
] can be decreased by adding Ag
+
(aq)
. The silver ion reacts with the thiocyanate anion to
form AgNCS
(s)
. The system described by reaction (8) responds to the decrease in [NCS
-
] by again shifting to
the reactant side to replace the depleted NCS
-
(aq)
until equilibrium is re-established.
In solution, [Fe(NCS)]
2+
(aq)
, has a rust (reddish) colour (in very dilute solutions, it may appear to be a lighter
gold or orange colour). The NCS
-
(aq)
, is colourless and Fe
3+
(aq)
is pale yellow. Hence, the colour of the solution
as well as the appearance of a precipitate can be used to monitor the position of equilibrium. If the rust colour
of the solution becomes more intense, this indicates an increase in the concentration of [Fe(NCS)]
2+
(aq)
complex
ion, caused by a shift in the position of equilibrium to the product side (i.e. to the right). However, if the rust
colour of the solution becomes less intense or a colour change occurs or if a precipitate forms, this would
indicate a decrease in the concentration of the [Fe(NCS)]
2+
(aq).
complex ion arising from a shift in the position of
equilibrium to the reactant side (i.e. to the left).