E-12
OBSERVATIONS & INFORMATION SHEET
- EXPERIMENT E
Le Châtelier's Principle and
Interactive Chemical Equilibrium
Part I. The Iron(III) Thiocyanate
Equilibrium
Write the balanced net
ionic equation for the equilibrium reaction between Fe(NO3)3
(aq)
and KNCS
(aq)
.
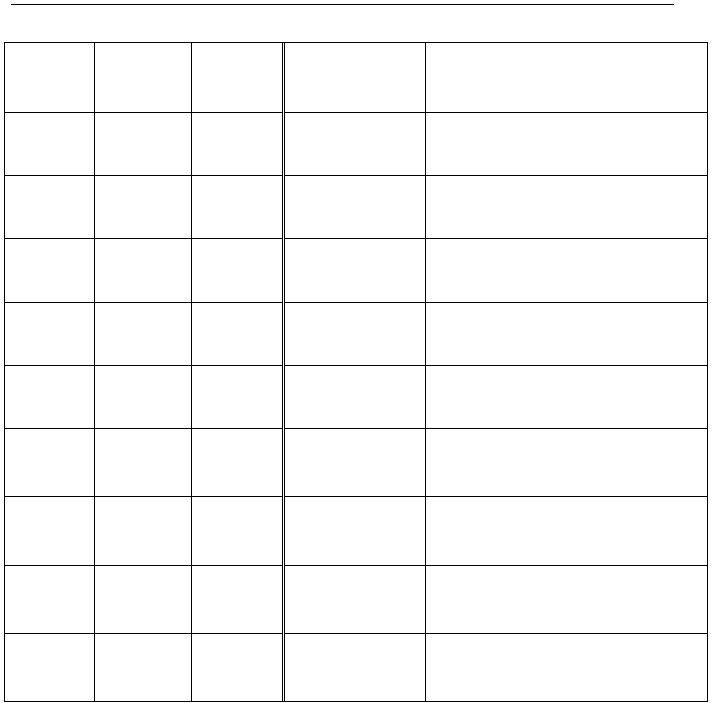
Observations for the addition of
various reagents to samples of the above equilibrium solution.
reagent(s)
added
description
of reactants
description of
reaction solution once
equilibrium is
re-established
i.
interactive equilibria product(s)
formed
ii.
the effect on the concentration
of
[Fe(NCS)]
2+
(aq)
Test Tube 1
5 drops H2O
colourless
liquid
medium rust-red
solution
i.
none
ii.
slight dilution of [Fe(NCS)]
2+
(aq)
***CONTROL - reference
colour
Test Tube 2
5 drops 0.10 M
Fe(NO3)
2(aq)
i.
______________________________________
ii.
______________________________________
Test Tube 3
5 drops 0.020 M
KNCS
aq)
i.
______________________________________
ii.
______________________________________
Test Tube 4
5 drops 6.0 M
NaOH
(aq)
i.
______________________________________
ii.
______________________________________
Test Tube 5
5 drops 0.20 M
NaF
(aq)
i.
______________________________________
ii.
______________________________________
Test Tube 6
2 drops 0.20 M
Na2C2O
4(aq)
i.
______________________________________
ii.
______________________________________
Test Tube 7
1 drop 0.10 M
K
4
[Fe(CN)
6
]
(aq)
i.
______________________________________
ii.
______________________________________
Test Tube 8
1 drop 1.0 M
NH
3(aq)
i.
______________________________________
ii.
______________________________________
Test Tube 9
5 drops 0.10 M
AgNO
3(aq)
i.
______________________________________
ii.
______________________________________
___________________________
_____________________________
_______________
Signature of Laboratory Instructor
Name of Student
Laboratory Section