D-7
OBSERVATIONS SHEET - EXPERIMENT D
*
DETERMINATION OF THE UNIVERSAL GAS CONSTANT
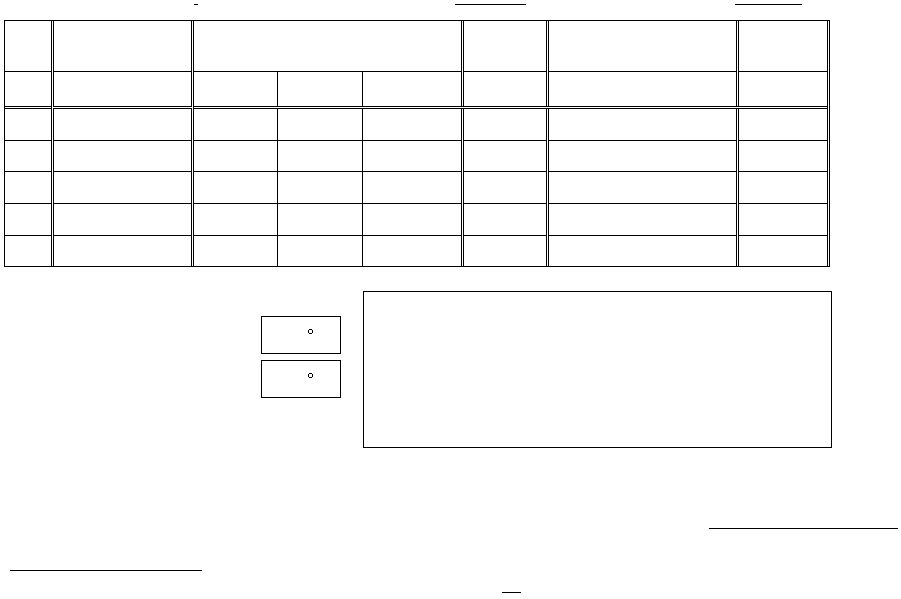
Barometric pressure: _________ (mm Hg)
Molarity of sulfamic acid:
M
Molarity of sodium nitrite:
M
Volume of
sulfamic acid solution
Volume of gas collected
(buret readings to 0.01 mL)
Room Temp
(t)
Vapor pressure
of water at room temp
(Show calculation in sapce provided)
Height of
water column
after reaction
Volume
(mL)
Initial
reading(mL)
Final
reading(mL)
Volume of N2*
(mL)
(°C)
(mm)
of Hg
h (mm)
Run 1
Run 2
Run 3
Run 4
Run 5
For Run 3
t1, temperature of solution
in the Erlenmeyer flask before reaction
C
t2, temperature of solution
in the Erlenmeyer flask after reaction
C
*Note: There is a small air space at the top of the buret not considered in this experiment. This dead volume can be ignored as the volume and pressure of the
air in it are nearly the same before and after the run. We will start measurements on the marked portion of the buret and ignore this dead volume.
____________________________
__________________________________________________
Signature of Laboratory Instructor
Name of Student
Laboratory Section
*
Reminder - Observation Sheets (and Report Sheets) MUST be completed in ink.
SHOW CALCULATION OF THE VAPOUR PRESSURE IN THIS SPACE