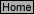
D-2
Figure 1
Tap wat
er
St
opcock
M
eniscus
Sulfamic
A
cid
Glass V
ial
containing NaNO2
U-Tube
250 mL
Erlenmey
er
Flask
400 mL
Beaker
Buret
Clamp
Tubing
Rubber
Stopper
P
atm
h
w
Fig
ure 2
P
gas
+
P
H
2
o
A correction must be made, if the height of the column of water (h) in the buret, after reaction, is different
from the level of water in the beaker. Consider Figure 2(above). The atmospheric pressure (P
atm
) pushes
down on the water in the beaker and forces water up into the buret. This external pressure is balanced by
the pressure of the N
2(g)
in the buret (P
N2
), the pressure of water vapor in the buret (P
H2O
), and the pressure
exerted by the column of water in the buret above the level of the water in the beaker (P
wc
). This balance
can be expressed as: P
atm
= P
N
2(g)
+ P
H2O
(g)
+ P
wc
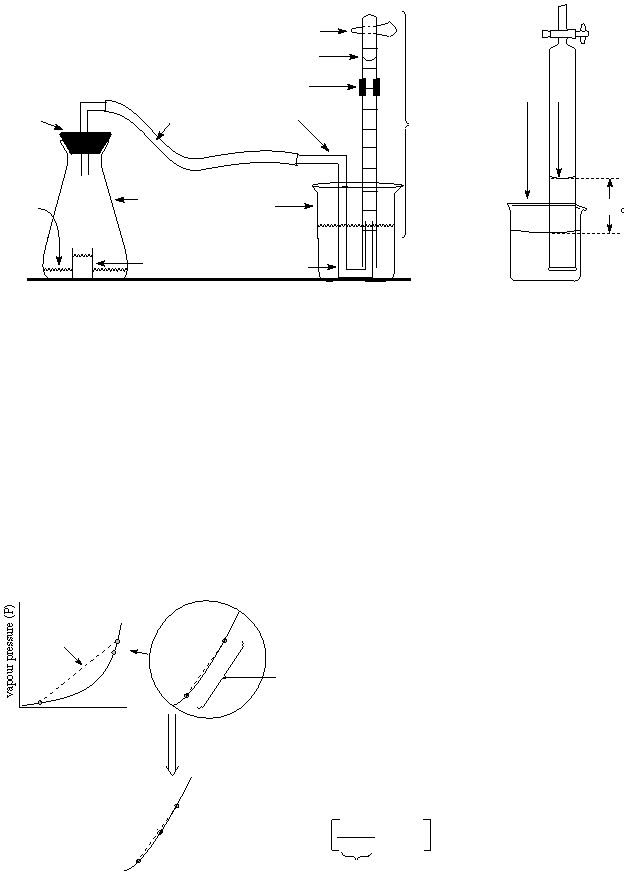
The vapour pressure at an untabluated temperature can be calculated using linear interpolation (even though
a plot of vapour pressure versus temperature is not linear). The diagram below illustrates how the method
of linear interpolation works and the calculation involved. You should try to understand how this method
works on your own. Your instructor will briefly go over the method.
A
B
C
B
A
Good linear i
nterpolation
Good ag
reement between line
a
nd g
raph, hence
may predict
val
ues be
twe
en points A and B
tempe
ra
ture(T)
Poor line
ar
interpolation
B(known value)
A
(known value)
D
(unknown value)
(P
A
-P
B
)
T
A
-T
B
(T
D
-T
B
)
x
sl
ope
P
D
=
P
B
+
P
A
is the va
por Pressure at poi
nt A
Where:
P
B
is the vapor Pressure at poi
nt B
P
D
is the vapor Pre
ssure at point D
T
A
is the tempe
rature at poi
nt A
T
B
is the temperature
at point B
T
D
is the tempe
rature at poi
nt D