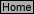
C-9
Table 1
Information to Identify Anions (Part III A.)
Anion
A.1:add
1 mL of 6.0 M HCl
A.2: add
1 mL of 6.0 M HCl and
1 mL of 1.0 M BaCl2
A.3: add
six drops of 0.10 M AgNO3
and one drop of 6.0 M HNO3
CO3²
-
gas evolved
no reaction
gas evolved & white ppt*
SO
4
2-
no reaction
white ppt*
ppt*
Cl
-
no reaction
no reaction
white curdy ppt*
Br
-
no reaction
no reaction
cream-colored ppt*
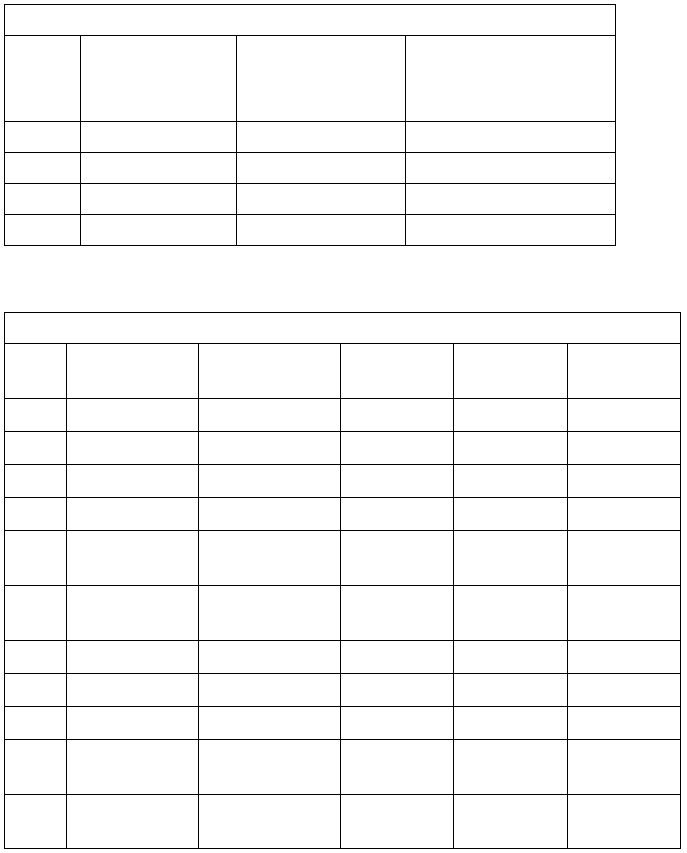
Table 2
Information to Identify Metal Cations (Part III B.)
Cation
B.1 : color of
aqueous solution
B.2 : add
3 drops NaOH
B.2: add
2 mL NaOH
B.3: add
6 drops H2SO
4
B.4 :
flame test
Cr
3+
blue
grey-green ppt*
ppt* dissolves
no reaction
-
V
2+
green
black ppt*
ppt* stays
no reaction
-
Cu
2+
blue
blue ppt*
ppt* stays
no reaction
-
Ni
2+
green
green ppt*
ppt* stays
no reaction
-
Na
+
colorless
no ppt*
no ppt*
no reaction
intense yellow-
orange flame
Mn
2+
colorless or pale
pink
white ppt* forms,
quickly turns tan
ppt* stays
no reaction
-
K
+
colorless
no ppt*
no ppt*
no reaction
violet flame
Al
3+
colorless
white ppt*
ppt* dissolves
no reaction
-
Mg
2+
colorless
trace of white ppt*
ppt* stays
no reaction
-
Co
2+
pink
blue ppt* forms, turns
pink-brown
ppt* stays
no reaction
-
Ba
2+
colorless
slight white ppt*
(traces on occasion)
ppt* stays
white ppt*