B-17
5.
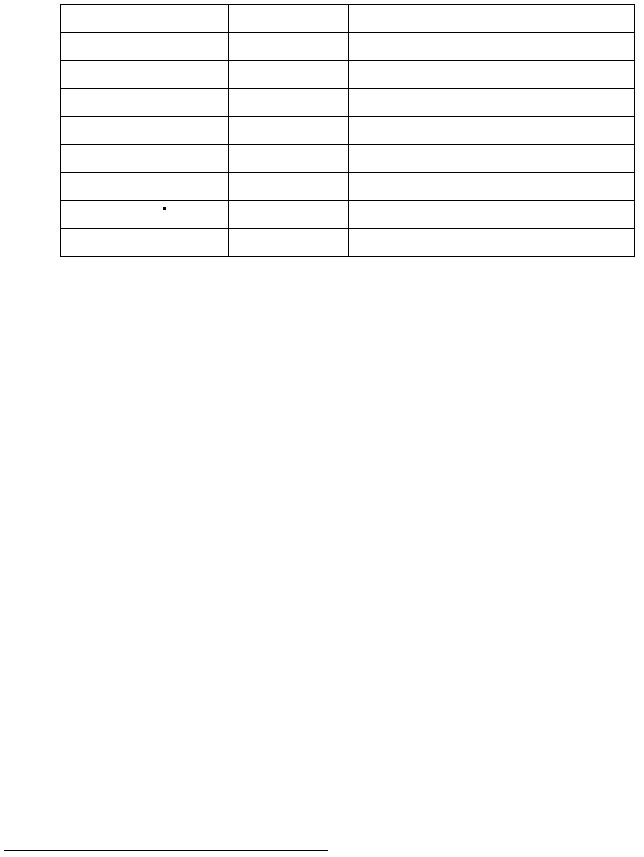
Complete the following table: (0.7 marks)
Compound
Color
Solubility in Water
Cu
rust red
insoluble
Cu(NO3)2
CuCO3
CuSO
4
Cu(OH)2
CuO
[Cu(NH3)
4
]SO
4
H2O
(s)
Cu
6.
Copper(II) oxide, CuO, may also be formed when copper metal is gently heated in air or oxygen
gas. Copper metal surfaces that have been tarnished by a layer of black CuO can be cleaned by
treating the surface with dilute solutions of hydrobromic acid (HBr(aq)). This process forms a
water soluble salt that can be washed away.
a)
Write the balanced molecular equation of the reaction that is responsible for the removal of
the CuO
(s)
from the copper metal surface. (0.5 marks)
b)
Is CuO an acidic or a basic oxide? Justify your answer. (0.5 marks)
ASSESSMENT
Performance
(1.0 mark):
Observation Sheet
(1.0 mark):
Quality of Copper metal
(1.5 marks):
Laboratory Report
(6.5 marks):
Deductions
(if any):