A-9
You must also turn in the original copy of the Observations Sheet to your laboratory instructor before
leaving. The second copy of the Observations Sheet should be kept in your laboratory manual for future
reference.
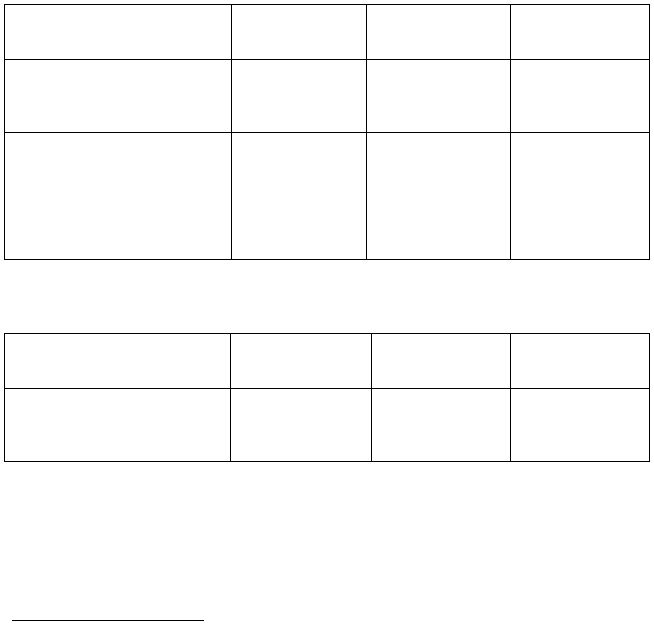
OBSERVATIONS & INFORMATION SHEET - EXPERIMENT A
*
Preparation of Copper(II) Sulfate
Part I:
mass of beaker and copper metal
_______________________________
mass of beaker
_______________________________
mass of copper metal
_______________________________
copper
reagent
reagent(s)
added
product(s)
formed
chemical formula:
CU(S)
HNO3(aq)
(CONCENTRATED)
description & observations:
(eg. color, solubility characteristics,
evolution of a gas, etc...)
Part II:
copper
reagent
reagent
added
product(s)
formed
chemical formula:
CU(NO3)2(aq)
Na2CO3(aq)