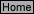N-8
water. This is an approximation, but a very good one for dilute solutions. The calculations might be
clearer if you keep separate what happens in the water, and what happens in the reaction. Generally,
the terms, system and surroundings, are used
when talking about heat flow. For our calorimeter,
the system is the chemical reaction, while the surroundings would be the solution in the
calorimeter. For an exothermic chemical reaction, the heat flows out of the system, (q
sys
is negative)
into the surroundings, so there is an increase in the temperature of the water, (q
surr
is positive)
The heat absorbed by the water in a calorimeter is given by:
q
surr
= q
p
= m x C
p
x (t
e
- t
i
) = m x C
p
x ?t
(17)
where m is the mass of the solution. The mass of solution is calculated by multiplying the volume of the
solution by its density which is assumed to be 1.02 g/mL for the Mg and MgO runs, and 1.00 g/mL for
parts II and III of the experiment. C
p
is the specific heat capacity of the water, t
e
is the extrapolated
temperature, and t
i
is the initial temperature. Because the calorimeter used is not a perfect insulator, the
corrected final temperature is obtained by extrapolation of the temperature versus time graph back to
time = 0, therefore, t
f
t
e
. This accounts for much of the heat lost by the calorimeter.
For a reaction in which the temperature of the water increases, q
surr
, the heat absorbed by the
surroundings (water), calculated by equation 17, is positive. However, for heat to be absorbed by the
surroundings, this heat must have been released by the system (reaction). Hence q
sys
must be the
negative of q
surr
:
q
sys
= - q
surr
?H
rxn
always describes the heat change for the reaction as written. Thus, for equation 7, it is the heat
change when one mole of MgO
(s)
is dissolved. To determine ?H
rxn
for equation 7, divide q
sys
by the
number of moles of MgO
(s)
used in the experiment. ?H is always expressed in kilojoules per mole,
kJ/mol.
In some cases you are asked to compare your experimental ?H
rxn
with the accepted value. The
percent error in your answer is defined as: