N-25
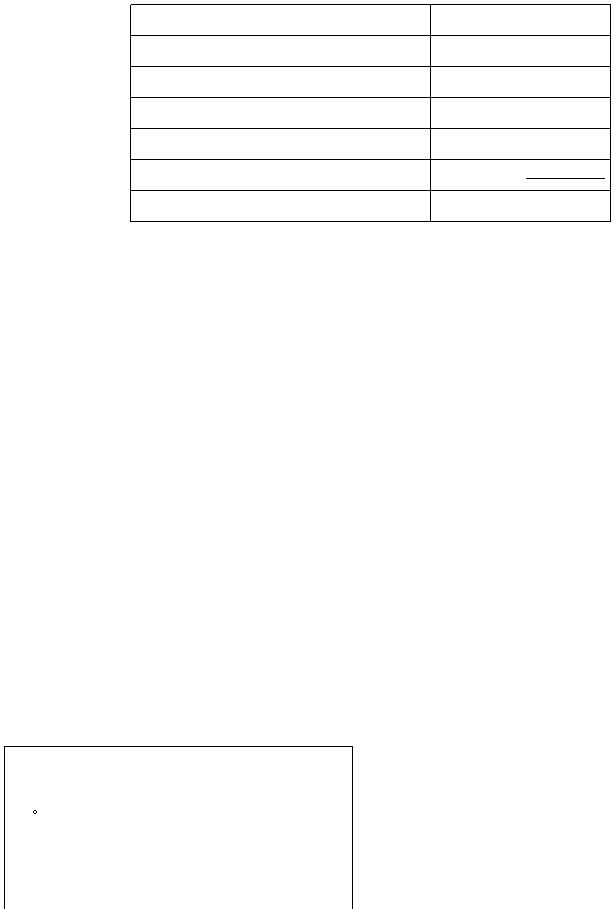
Results(Part III b)
Volume of CH3COOH
mL
Volume of NaOH
mL
Assumed volume of water
mL
?t
°C
Heat absorbed by water
J
Amount of limiting reagent (specify reagent)
mol
?H
overall
per mol of water formed
kJ/mol
A.
Calculate the heat absorbed by the water in the calorimeter due to this reaction.
(0.2 marks)
B.
Calculate the ?H
overall
in kJ/mol of water formed. (0.5 marks)
C.
Calculate the ?H
dissociation
of CH3COOH. Include appropriate net ionic equations
(0.4 marks)
ASSESSMENT
Performance
(1.0 mark):
?H
f
of H2O
(l)
(1.0 mark):
Laboratory Report
(8.0 marks):