N-18
REPORT: EXPERIMENT N - ENTHALPY OF REACTION
Name ____________________________________
Section ___________ Date ___________________
Laboratory Instructor________________________
GRAPHS (1.5 marks) Plot the graphs accurately on graph paper that has a 1.0 mm grid.
NOTE: When determining the scales for your graphs, be sure to use the largest
possible scale that is convenient and take into account the accuracy of your data.
Title each graph accurately (i.e. standard format).
Draw the best-fit straight line through your data points to determine t
e
.
Include units and use the correct number of significant figures in all calculations.
Part I. Determination of Standard Heat of Formation, ?H
f
, of H2O
(l)
A. Determination of ?H
6
: (Your graph for this part must be attached.)
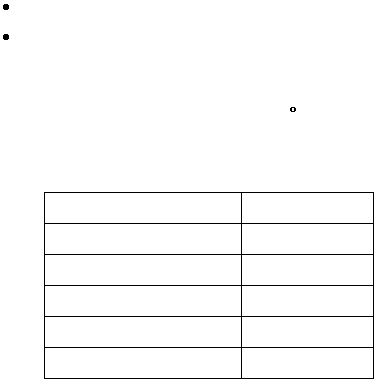
Results
Volume of HCl
mL
Mass of Mg
g
?t
°C
Heat absorbed by water
J
Amount of magnesium
moles
?H
6
per mol of Mg
kJ/mol
Show detailed calculations for the heat absorbed by the water in this reaction, q
surr
, and of ?H
6
. Include
the calculations for ?t. (1.0 mark)