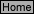V-8
(5.0)
14.
(Exp R) When studying the Nernst equation, students were asked to determine the formation
constant, K
f
(Kst), of the complex ion, [Zn(OH)
4
]²
-
(aq)
. To do this, a solution was prepared in
which the initial concentrations were 1.00 x 10
-2
M Zn(NO3)2, zinc nitrate, and 5.00 M
NaOH, sodium hydroxide. The reaction was allowed to reach equilibrium with the
formation of [Zn(OH)
4
]²
-
(aq)
. To determine the equilibrium concentration of uncomplexed
Zn
2+
(aq)
ions remaining in the reaction solution at the end of the complexation reaction, a
voltaic cell was set up.
The anode half-cell was made using the [Zn(OH)
4
]²
-
(aq),
Zn
2+
(aq)
, OH
-
(aq)
reaction solution
with a zinc metal electrode. The cathode was a Zn
2+
(1.00 M)/Zn reference half-cell. The
measured (non-standard) cell voltage was 0.650 V.
i)
Write the balanced net ionic equation for the formation of [Zn(OH)
4
]²
-
(aq)
.
ii)
Using the Nernst equation and the E
cell
, calculate the equilibrium [Zn
2+
]
(aq)
in the
reaction solution.
iii)
Calculate the formation constant, K
f
, for [Zn(OH)
4
]²
-
(aq)
.