V-6
(1.0)
12.
(Exp T) The activation energy, E
a
, can be obtained using the equation:
ln k = -
R
,
Ea
1
T
+ ln A
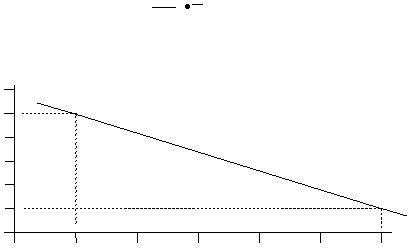
From the following graph of student data, determine E
a
.
a.
-3.6 x 10³ J mol
-1
b.
-3.0 x 10
4
J mol
-1
c.
2.3 x 10
-3
J mol
-1
d.
3.0 x 10
4
J mol
-1
e.
3.7 x 10
4
J mol
-1
0.0031
1.8
-4.6
-
4.1
-3.7
-3.2
-
2.8
-
2.3
1/T (K)
ln k
0.0033
0.003
5