U-2
element is cesium. In a simple covalent compound, such as I-Cl, the more electronegative element Cl (
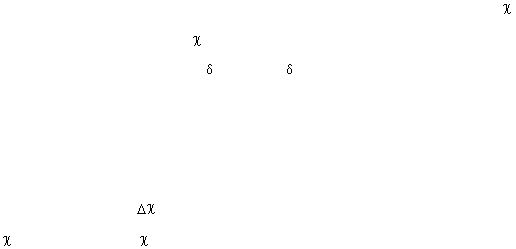
=3.0) will
attract the bonding electrons more strongly than I (
=2.5), resulting in a polarization of the bond as shown below:
(
+) I -- Cl (
-)
As the electronegativity difference between the two elements becomes larger, the bond between them becomes
more polarized until the bonding pair of electrons is no longer shared but instead localized on the more
electronegative element. At this point the bonding is mainly ionic. As a general rule-of-thumb, the bonding in a
binary compound composed of a metal and a non-metal can be described as ionic if the electronegativity
difference between the two elements (
) is greater than 2.0. For instance, the electronegativity difference
between sodium (
= 0.9) and chlorine (
= 3.0) is 2.1; therefore, NaCl is classified as an ionic compound. By
contrast, a bond is considered to be covalent if the electronegativity difference between the two bonded atoms is
less than 0.5. If the difference in electronegativity is between these values, the bond is classified as polar
covalent.
Solubility and Intermolecular Forces
The solubility of a substance is the amount of the substance that will saturate a specified amount of solvent at a
given temperature. Substances will dissolve in each other only if the intermolecular forces in the solution are
similar to those in the separate components. Put another way, “like dissolves like” or “oil and water do not mix”.
For example, non-polar substances such as oil, gasoline, benzene dichloromethane and dichloromethane mix well
with each other, but they do not dissolve in highly polar or ionic substances such as water or sodium chloride. By
contrast, polar or ionic compounds are insoluble in non-polar substances, but readily dissolve in polar solvents, such
as water.
Water is the solvent most frequently employed by chemists. Water molecules are closely associated with each
other through hydrogen bonding, and compounds that form hydrogen bonds tend to be soluble in water. For
instance, relatively small, hydrogen-bonded molecules, such as ethanol, are completely miscible in water (i.e. mix in
all proportions).
Water is referred to as an ionizing solvent because of its ability to stabilize ions in solution. Water dissolves ionic
compounds by surrounding each ion with a sheath of water molecules, called a hydration layer. The layers of
water help to pull the ions out of the crystal lattice and prevent them from reassembling once they are in solution.
The attraction between an ion and a polar molecule is called an ion-dipole interaction. Similarly, the attraction
between two (or more) polar molecules is called a dipole-dipole interaction.