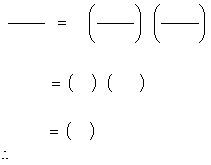
S-6
The results for runs 1 and 3 will allow us to solve for the exponent, m:
m = 1
For this example, the results show that the rate depends on [A]¹ and [B]².
III. Reaction Mechanism
Most chemical reactions do not occur in a single step. For example, in the reaction shown by
equation (4), the stoichiometry requires that three molecules, exactly one of A and two of B, collide at
the same time. However, experiments of gas phase reactions have shown that these three-body
collisions are unlikely. It is more likely that the reaction proceeds by a series of steps. Such a sequence
of steps is called a reaction mechanism. Mechanisms cannot be proven absolutely. They are
models of what we think is occurring at the molecular level. The most we can say is that a mechanism is
consistent with our experimental results. In proposing a reaction mechanism, two conditions must be
satisfied:
a)
the overall sum of the proposed steps in the reaction mechanism must satisfy the balanced
stoichiometric equation for the reaction.
b)
the rate equation derived by assuming that a particular step is rate determining (slowest) agrees
with an experimentally determined rate equation.
2
m
010
.
0
015
.
0
010
.
0
017
.
0
010
.
0
038
.
0
25
.
2
7
.
1
8
.
3
m
m
7
.
1
7
.
1