R-15
REPORT: EXPERIMENT R - THE NERNST EQUATION
AND ITS APPLICATIONS
Name ____________________________________
Section ___________ Date ___________________
Laboratory Instructor________________________
Please show all calculations with units and the correct number of significant figures.
Note that metal ion concentrations can be calculated to three significant figures if you had measured the
voltages to 0.001V.
Part I. The Effect of Concentration on Electrode Potentials
A.
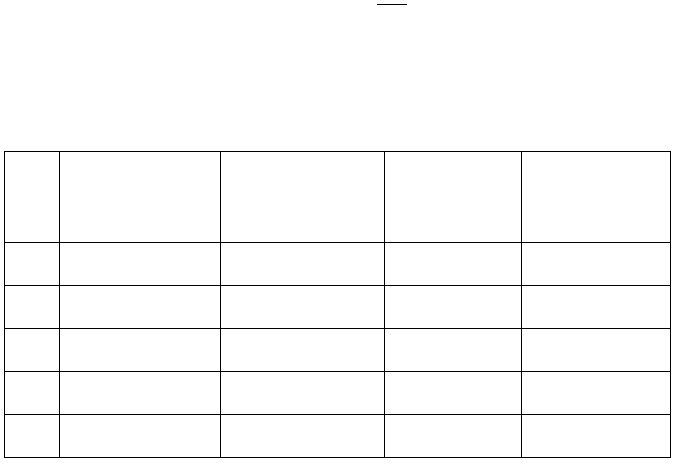
Fill in the following Table including all calculations: (0.8 mark)
[Ag
+
]
(M)
log [Ag
+
]
(M)
Measured cell
potential
(volts)
Calculated half-cell
potential*, E
ox
(volts)
set 1
1.00 M
set 2
1.00 x 10
-1
M
set 3
1.00 x 10
-2
M
set 4
1.00 x 10
-3
M
set 5
1.00 x 10
-4
M
*
This is the potential of the test solution based on E
cell
= E
red, cath
- E
red, anode
where the reference half-
cell is assigned a value of 0.800 volts and E
cell
is the value you measured in your experiment.