R-13
OBSERVATIONS SHEET - EXPERIMENT R
THE NERNST EQUATION AND ITS APPLICATIONS
NOTE: Record all cell potentials to 3 decimal places.
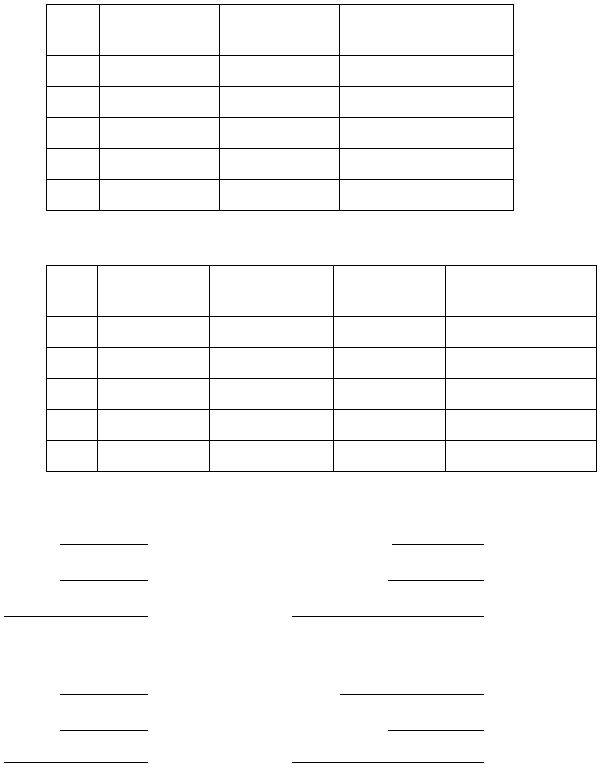
Part I: The Effect of Concentration on Electrode Potentials
[Ag
+
]
in test half-cell
E
cell
(V)
cathode
1
1.00 x 10
-4
M
2
1.00 x 10
-3
M
3
1.00 x 10
-2
M
4
1.00 x 10
-1
M
5
1.00 M
0.000 (assumed)
Ag
(s)
in 1.00 M solution
Part II: The Determination of an Equilibrium Constant
[Ag
+
]
in test half-cell
[Cu
2+
]
in reference cell
E
cell
(V)
cathode
1
1.00 x 10
-4
M
1.00 M
2
1.00 x 10
-3
M
1.00 M
3
1.00 x 10
-2
M
1.00 M
4
1.00 x 10
-1
M
1.00 M
5
1.00 M
1.00 M
Part III: The Determination of a Solubility Product (K
sp
)
The [NaOH]:
Volume of NaOH used:
The [CuSO
4
]:
Volume of CuSO
4
used:
E
cell
:
Cathode:
Part IV: The Determination of a Formation Constant (K
f
)
The [NH3]:
Volume of NH3 used:
The [CuSO
4
]:
Volume of CuSO
4
used:
E
cell
:
Cathode: